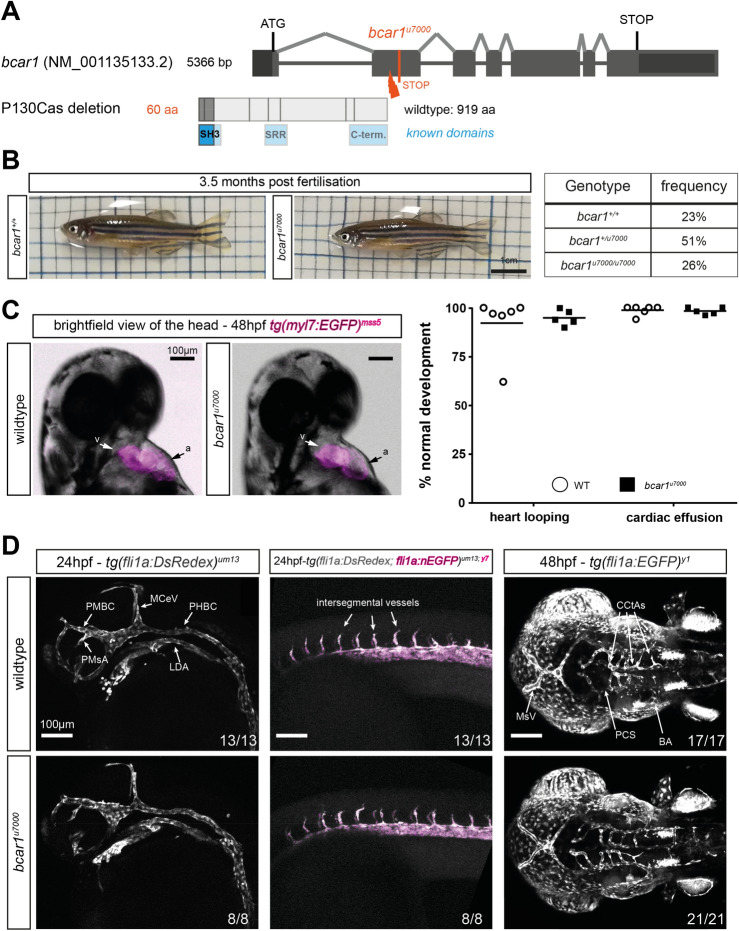
Figure 1.
Loss of P130Cas does not induce lethality, major morphological defects, or defects in early arterial angiogenesis in zebrafish. (A) Schematic showing the exon–intron structure of bcar1 and location of the genetic insertion-deletion of bcar1u7000 resulting in protein truncation during translation, known protein domains are indicated in blue. (B) Photographs of live adults (left) and diagramme showing actual observed viability from a bcar1u7000/+ heterozygous incross (right). Homozygous deleted fish are observed with expected Mendelian frequency; morphologically, they appear indistinguishable from wildtype siblings. (C) Brightfield image with fluorescent overlay of tg(myl7:EGFP)mss5 zebrafish hearts at 48hpf (left), clearly showing ventricle (v) and atrium (a). Quantification of n = 6 wildtype and n = 5 bcar1u7000 clutches with n ≥ 53 embryos each (right) confirm the absence of heart looping or effusion (oedema) defects at 48hpf. (D) Maximum intensity projections, lateral views of the head (left column) and trunk (middle column) at 24hpf shows emergence of primordial midbrain channel (PMBC), middle cerebral vein (MCeV), primordial hindbrain channel (PHBC), lateral dorsal aorta (LDA), primitive mesencephalic artery (PMsA), and intersegmental vessel sprouting as normal in bcar1u7000 mutants. Dorsal to ventral view shows emergence of cerebral central arteries (CCtAs), basilar artery (BA) and posterior communicating segment (PCS) by 48hpf as normal (right column). Experimental n as indicated per image, from ≥ 2 independent clutches. Scale bars as indicated.