Figure 4.
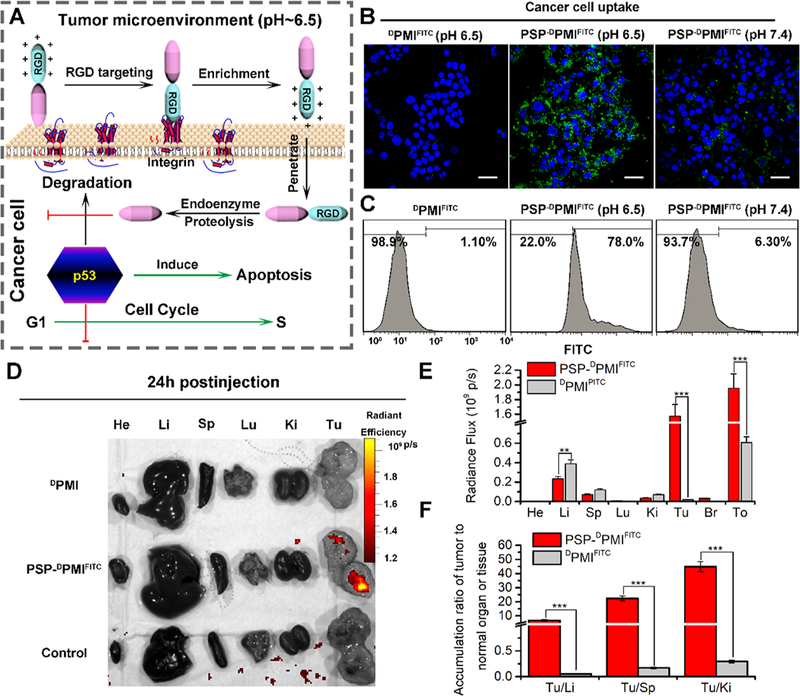
PSP-based self-assembly endows the DPMI with cytomembrane penetrability, long circulation time, and tumor targeting. (A) Schematic depiction for targeting function of RGD motif and the response of PSP-DPMI to tumor extracellular pH. (B) CLSM images of HCT116 cells after 6 h of incubation with DPMIFITC or PSP-DPMIFITC (20 μg/mL) at pH 7.4 or pH 6.5 (scale bar: 60 μm). All photos were taken at the same exciting light and detector gain. (C) Flow cytometry analysis was performed to measure cell uptake of DPMIFITC or PSP-DPMIFITC responded to acidic pH. (D) Ex vivo fluorescent images of tumors and major organs from PMI - and PSP- PMI -treated mice at 12 h postinjection. He, heart; Li, liver; Sp, spleen; Lu, lung; Ki, kidneys; Tu, tumor; Br, brain; To, total. Thresholds were appropriately established: 5.0 × 108. (E) Quantitative analysis of the Ex vivo fluorescence intensity in tumors and major organs. Every tissue was exposed at the same exciting light and normalized by the PBS control group. All data are shown by mean ± s.d. (n = 3/group). (F) Tumor-to-background (normal organ or tissue) ratios for DPMIFITC and PSP-DPMIFITC at 24 h postinjection. PSP-DPMIFITC showed improved tumor selectivity over the surrounding normal organs or tissues compared to DPMIFITC (n = 3). p values were calculated by t-test (**, p < 0.01; ***, p < 0.001).
