Abstract
Background
This study examined the impact of mitral valve repair (MVRe) on survival of patients with moderate or severe (≥2+) MR and ischemic cardiomyopathy randomized to coronary artery bypass grafting (CABG) versus CABG+surgical ventricular reconstruction (SVR) in the STICH trial.
Methods
Among patients with moderate or severe MR and ischemic cardiomyopathy undergoing CABG or CABG+SVR, the impact of MVRe on mortality between the two treatment arms was compared.
Results
Among 867 patients with assessment of baseline MR severity, 211 had moderate or severe MR. After excluding 7 patients who underwent mitral valve replacement, 50, 44, 62, and 48 patients underwent CABG, CABG+MVRe, CABG+SVR, and CABG+SVR+MVRe, respectively. Four-year mortality rates were lower following CABG+MVRe than CABG alone (16% vs. 55%; adjusted hazard ratio [HR] 0.30; 95% CI 0.13–0.71). In contrast, the CABG+SVR+MVRe and CABG+SVR groups had similar 4-year mortality of 39% vs. 39% (adjusted HR 0.88; 95% CI 0.46–1.70). MVRe had a more favorable effect on survival in patients undergoing CABG alone compared to CABG+SVR (p=0.013). Baseline MR severity was similar between patients that received CABG+MVRe and those that underwent CABG+SVR+MVRe. A larger proportion of patients demonstrated a reduction in MR between 4 and 24 months after CABG+MVRe compared to CABG+SVR+MVRe (50.0% versus 25.0%, p=0.023).
Conclusion
In patients with moderate or severe MR and ischemic cardiomyopathy undergoing CABG, MVRe appears to have a favorable effect on survival. The addition of SVR to CABG may attenuate the anticipated benefits of MVRe by limiting the long-term reduction of MR with MVRe.
Keywords: mitral regurgitation, mitral valve repair, heart failure, left ventricular dysfunction, ischemic cardiomyopathy, coronary artery bypass grafting, surgical ventricular reconstruction
Introduction
Left ventricular (LV) remodeling as a result of ischemic heart disease is associated with progression of heart failure and poor clinical outcomes 1. Surgical ventricular reconstruction (SVR) reduces LV volume and improves LV ejection fraction in patients with LV remodeling resulting from coronary artery disease 2. The Surgical Treatment for Ischemic Heart Failure (STICH) study was conducted to examine 1) whether coronary artery bypass grafting (CABG) improves outcomes when added to medical therapy (Hypothesis 1) and 2) whether the addition of SVR to CABG improves outcome in ischemic cardiomyopathy (Hypothesis 2) 3. While SVR significantly reduced LV volume when compared with CABG alone, it did not improve clinical outcomes 4. In addition, studies have demonstrated that worsening of mitral regurgitation (MR), diastolic function, and sphericity is associated with the combined procedure 5–9. In the Hypothesis 1 arm of the STICH trial, baseline MR severity was strongly associated with mortality in patients with ischemic cardiomyopathy treated with medical therapy alone 10, and in patients with moderate or severe MR, concomitant mitral valve repair (MVRe) may improve long-term survival compared with CABG alone 10. In contrast, The Cardiothoracic Surgical Trials Network (CSTN) reported that the addition of MVRe to CABG among those with moderate ischemic MR led to a reduction in the prevalence of moderate or severe MR but not mortality or hospital readmission at 1- and 2-years follow-up 11. Since the benefit of adding mitral valve (MV) surgery to CABG in patients with significant MR has remained unclear, we sought to examine the impact of MVRe on survival of patients with ischemic cardiomyopathy and moderate or severe MR at baseline who underwent CABG or CABG+SVR in the Hypothesis 2 component of the STICH trial.
Materials and Methods
Patient selection
The experimental design of the STICH trial have been previously described 3,4. Between September 2002 and January 2006, 1000 patients with coronary artery disease, LVEF ≤ 35%, and dominant anterior akinesia/dyskinesia amenable to CABG and SVR were randomized to CABG alone (499 patients) or CABG+SVR (501 patients). Eligibility for SVR was determined clinically, and no definite cut-off value for LV volume was specified for enrolment. MR severity assessed by the echocardiography core laboratory was not available to the study surgeon, and the decision to perform adjunctive MV repair or replacement was left to the surgeon’s discretion. For this study, 7 patients with moderate or severe MR who underwent mitral valve replacement were excluded from outcome analysis.
Echocardiographic measurements
Transthoracic echocardiography was performed at baseline, 4 months and 2 years following enrolment. Echocardiographic studies were analyzed by the echocardiography core laboratory without knowledge of clinical data according to the recommendations from the American Society of Echocardiography 12–14. The severity of MR was assigned a numeric grade of 0 (none or trivial), 1 (mild), 2 (moderate), 3 (moderate-severe), 4 (severe), or 5 (indeterminate) by integrating qualitative (visual assessment of the width, depth, and area of the MR jet) and quantitative parameters.
Statistical analyses
Baseline characteristics were described using mean±standard deviation for continuous variables and frequency (percentage) for categorical variables. Group comparisons of continuous and ordinal baseline variables were performed using Kruskal-Wallis non-parametric analysis of variance, while categorical variables were compared using the chi-square test. Intention-to-treat analysis was used as the methodology for this report. Mortality rates as a function of time from randomization and randomized treatment arm were estimated using the Kaplan-Meier method 15. Overall mortality differences between patient groups were assessed using the Cox proportional hazards model 16. The Cox model was also used to assess the impact of MVRe on mortality, adjusting for significant clinical factors that could affect the decision (propensity) for having the MV procedure or that might reflect differences in the prognosis of patients who did versus did not undergo MV surgery. As a preliminary step to these analyses, multivariable logistic regression, using both forward (stepwise) and backward variable selection algorithms, was used in the overall Hypothesis 2 population to identify clinical variables that were significantly associated with whether patients received MV surgery. Candidate variables for the logistic analysis included demographics, comorbidities, risk factors, severity of coronary disease and LV function, selected laboratory measures, baseline six-minute walk distance, and the geographic region where the patients were enrolled.
In patients randomized to the CABG or CABG+SVR treatment arms who underwent concurrent MVRe, the pattern of change in MR over time was examined using the Wilcoxon rank-sum test.
All analyses were performed using SAS version 9.2 and above (Cary, NC), and a p value <0.05 was considered statistically significant.
Results
Study population
Of the 1000 patients in the Hypothesis 2 arm, 867 (mean age 61.7±9.8 years, 84.7% male) had echocardiography core laboratory assessment of baseline MR severity – 221(25.5%) as grade 0 MR, 435 (50.2%) as grade 1, and 211 (24.3%) as grade 2–4. These patients were randomized to the CABG (433 patients) or CABG+SVR (434 patients) treatment arms. Baseline characteristics of patients categorized according to MR severity at baseline are shown in Table 1. There was no significant difference in comorbidities among patients with different degrees of MR. Increasing MR grade was associated with larger LV end-diastolic and end-systolic volume index, lower LVEF, larger left atrial volume index, higher mitral-inflow E/A ratio, higher E/e’ ratio, and higher pulmonary artery systolic pressure (Table 2).
Table 1.
Baseline characteristics of STICH trial hypothesis 2 patients by mitral regurgitation severity.
| Characteristics | Baseline MR Severity | p-value | ||
|---|---|---|---|---|
| Grade 0 (N=221) | Grade 1 (N=435) | Grade 2–4 (N=211) | ||
| Age (year) | 60.1±10.1 | 62.4±9.6 | 62.1±9.8 | 0.011 |
| Male (%) | 197 (89.1) | 373 (85.7) | 164 (77.7) | 0.003 |
| White | 205 (92.8) | 391 (89.9) | 186 (88.2) | 0.261 |
| Body Surface Area ( m2) | 2.0±0.2 | 1.9±0.2 | 1.9±0.2 | <0.001 |
| Comorbidities | ||||
| Myocardial infarction | 193 (87.3) | 384 (88.3) | 183 (86.7) | 0.842 |
| Hyperlipidemia | 168 (76.4) | 316 (72.8) | 145 (69.0) | 0.234 |
| Hypertension | 125 (56.6) | 259 (59.5) | 127 (60.2) | 0.699 |
| Diabetes | 71 (32.1) | 158 (36.3) | 64 (30.3) | 0.266 |
| Chronic renal insufficiency | 13 (5.9) | 38 (8.7) | 18 (8.5) | 0.425 |
| Stroke | 15 (6.8) | 26 (6.0) | 10 (4.7) | 0.660 |
| Previous PCI | 51 (23.1) | 83 (19.1) | 36 (17.1) | 0.268 |
| Prior CABG | 4 (1.8) | 13 (3.0) | 5 (2.4) | 0.652 |
| NYHA heart failure class | <0.001 | |||
| I | 17 (7.7) | 20 (4.6) | 3 (1.4) | |
| II | 68 (30.8) | 111 (25.5) | 54 (25.6) | |
| III | 109 (49.3) | 198 (45.5) | 110 (52.1) | |
| IV | 27 (12.2) | 106 (24.4) | 44 (20.9) | |
| Angiographic results | ||||
| LM stenosis (≥50% stenosis) | 36 (16.3) | 90 (20.7) | 36 (17.1) | 0.309 |
| Proximal LAD (≥75% stenosis) | 158 (71.5) | 335 (77.0) | 157 (74.8) | 0.302 |
Values in table are mean ± standard deviation (SD) or n (%).
CABG, coronary artery bypass grafting; LAD, left anterior descending artery; LM, left main coronary artery; MR, mitral regurgitation; NYHA New York Heart Association; PCI, percutaneous coronary intervention.
Table 2.
Baseline echocardiographic parameters of STICH Trial Hypothesis 2 patients by mitral regurgitation severity.
| Characteristics | Baseline MR Severity | p-value | ||
|---|---|---|---|---|
| Grade 0 (N=221) | Grade 1 (N=435) | Grade 2–4 (N=211) | ||
| LVEF (%) (n=671) | 32.4±8.5 | 29.5±7.8 | 26.9±7.7 | <0.001 |
| LVEDV index (mL/m2) (n=671) | 106.3±32.5 | 114.8±33.2 | 130.1±40.6 | <0.001 |
| LVESV index (mL/m2) (n=671) | 73.1±27.9 | 82.0±29.0 | 96.3±35.7 | <0.001 |
| LA volume index (mL/m2) (n=546) | 34.0±9.9 | 41.5±13.0 | 50.9±19.6 | <0.001 |
| E/A ratio (n=689) | 0.9±0.6 | 1.3±0.9 | 1.8±1.0 | <0.001 |
| E/e' septal (n=396) | 12.7±4.9 | 16.7±8.7 | 22.2±12.0 | <0.001 |
| Estimated PA systolic pressure (mmHg) (n=197) | 33.0±9.0 | 41.4±14.9 | 46.2±15.1 | <0.001 |
Values in table are mean ± standard deviation (SD).
LA, left atrial; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MR, mitral regurgitation; PA; pulmonary artery.
Impact of mitral valve surgery on survival of patients with moderate or severe mitral regurgitation at baseline
Of the 211 patients with moderate or severe (grade 2–4) MR at baseline, 7 patients (6 patients in the CABG treatment arm and 1 patient in the CABG+SVR arm) received MV replacement, and they were excluded from the analyses below. Among 94 patients with moderate or severe MR at baseline and randomized to CABG, 50 patients underwent CABG only, while 44 patients underwent concurrent MVRe. Among 110 patients with moderate or severe MR who were randomized to CABG+SVR, 48 underwent concomitant MVRe and 62 did not. There were no significant differences in the demographic characteristics among these 4 subgroups (Table 3). Seven patients (6 patients in the CABG treatment arm and 1 patient in the CABG+SVR arm) received MV replacement for moderate or severe MR at baseline, and they were excluded from the analyses below.
Table 3.
Baseline characteristics of STICH trial hypothesis 2 patients with moderate or severe mitral regurgitation at baseline.
| Characteristics | Patient group | p-value | |||
|---|---|---|---|---|---|
| CABG only (N=50) | CABG + MVRe (N=44) | CABG + SVR (N=62) | CABG + SVR + MVRe (N=48) | ||
| Age (year) | 61.9±9.9 | 60.9±9.4 | 63.2±9.5 | 61.9±10.7 | 0.668 |
| Male (%) | 37 (74.0) | 36 (81.8) | 46 (74.2) | 40 (83.3) | 0.541 |
| White | 46 (92.0) | 37 (84.1) | 53 (85.5) | 44 (91.7) | 0.492 |
| Body Surface Area ( m2) | 1.9±0.2 | 1.9±0.2 | 1.9±0.3 | 1.9±0.2 | 0.337 |
| Comorbidities (%) | |||||
| Myocardial infarction | 45 (90.0) | 42 (95.5) | 52 (83.9) | 39 (81.3) | 0.158 |
| Hyperlipidemia | 35 (70.0) | 29 (67.4) | 46 (74.2) | 29 (60.4) | 0.483 |
| Hypertension | 37 (74.0) | 24 (54.5) | 37 (59.7) | 24 (50.0) | 0.085 |
| Diabetes | 16 (32.0) | 10 (22.7) | 23 (37.1) | 13 (27.1) | 0.416 |
| Chronic renal insufficiency | 5 (10.0) | 5 (11.4) | 7 (11.3) | 1 (2.1) | 0.250 |
| Stroke | 3 (6.0) | 2 (4.5) | 2 (3.2) | 2 (4.2) | 0.965 |
| Previous PCI | 8 (16.0) | 10 (22.7) | 8 (12.9) | 9 (18.8) | 0.597 |
| Prior CABG | 1 (2.0) | 1 (2.3) | 1 (1.6) | 0 (0.0) | 0.893 |
| Symptom status at baseline CCS angina class |
0.287 | ||||
| No angina | 11 (22.0) | 9 (20.5) | 20 (32.3) | 14 (29.2) | |
| I | 0 (0.0) | 4 (9.1) | 1 (1.6) | 7 (14.6) | |
| II | 9 (18.0) | 13 (29.5) | 13 (21.0) | 6 (12.5) | |
| III | 26 (52.0) | 14 (31.8) | 26 (41.9) | 18 (37.5) | |
| IV | 4 (8.0) | 4 (9.1) | 2 (3.2) | 3 (6.3) | |
| NYHA heart failure class | 0.274 | ||||
| I | 2 (4.0) | 1 (2.3) | 4 (6.5) | 3 (6.3) | |
| II | 25 (50.0) | 20 (45.5) | 16 (25.8) | 18 (37.5) | |
| III | 22 (44.0) | 20 (45.5) | 40 (64.5) | 24 (50.0) | |
| IV | 1 (2.0) | 3 (6.8) | 2 (3.2) | 3 (6.3) | |
| Six-minute walk test | |||||
| Able to perform | 37 (75.5) | 31 (70.5) | 48 (78.7) | 34 (70.8) | 0.731 |
| Distance walked (meter) | 291.9±132.1 | 307.3±113.2 | 333.4±99.0 | 331.0±115.6 | 0.330 |
| Angiographic results | |||||
| LM stenosis (≥50% stenosis) | 6 (12.0) | 10 (22.7) | 10 (16.1) | 8 (16.7) | 0.581 |
| Proximal LAD (≥75% stenosis) | 37 (75.5) | 31 (70.5) | 45 (72.6) | 37 (77.1) | 0.886 |
| Echocardiographic results | |||||
| LVEF (%) | 27.9±8.9 | 27.1±7.9 | 27.2±7.3 | 25.4±7.0 | 0.486 |
| LVEDV index (mL/m2) | 118.1±35.6 | 138.2±46.3 | 128.2±39.6 | 134.1±41.2 | 0.240 |
| LVESV index (mL/m2) | 86.7±33.5 | 103.0±42.4 | 94.4±33.6 | 100.6±34.9 | 0.191 |
| LA volume index (mL/m2) | 45.8±18.0 | 56.8±22.2 | 49.9±21.0 | 50.7±15.6 | 0.102 |
| Septal E/e' | 19.9±9.4 | 24.8±9.1 | 20.7±13.7 | 21.9±10.8 | 0.203 |
| PASP (mmHg) | 40.8±15.7 | 51.8±16.8 | 44.0±13.6 | 51.1±15.9 | 0.171 |
| Mitral regurgitation grade | |||||
| Grade 2 | 42 (84.0) | 20 (45.5) | 46 (74.2) | 26 (54.2) | <0.001 |
| Grade 3 | 7 (14.0) | 15 (34.1) | 15 (24.2) | 11 (22.9) | |
| Grade 4 | 1 (2.0) | 9 (20.5) | 1 (1.6) | 11 (22.9) | |
Values in table are mean ± standard deviation (SD) or n (%).
CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; LA, left atrial; LAD, left anterior descending artery; LM, left main coronary artery; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MR, mitral regurgitation; MVRe, mitral valve repair; NYHA New York Heart Association; PASP, pulmonary artery systolic pressure; PCI, percutaneous coronary intervention; SVR, surgical ventricular reconstruction.
In the 50 patients that underwent CABG alone, there were 21 deaths (42%) during follow-up, compared with 10 deaths (23%) among the 44 patients who underwent concomitant MVRe. Among the 48 patients with moderate or severe MR at baseline randomized to CABG+SVR who underwent concurrent MVRe, there were 20 deaths (42%) compared to 21 deaths (34%) among the 62 patients who did not undergo MVRe.
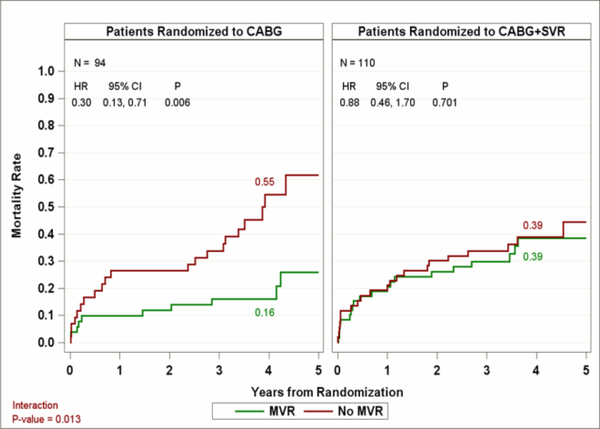
Since MVRe was not a randomized treatment, mortality comparisons of patients who did vs. did not undergo MVRe were adjusted for factors related to whether MV surgery was performed, as identified by logistic regression propensity analysis. These factors included site-reported MR severity, baseline LV end-systolic volume index, and baseline hemoglobin. Patients with moderate or severe baseline MR who underwent CABG without MVRe had a significantly higher adjusted 4-year mortality rate of 55%, compared with 16% in those who received CABG+MVRe (adjusted HR 0.30; 95% CI 0.13–0.71) (Figure 1). In contrast, both the CABG+SVR and the CABG+SVR+MVRe groups had an adjusted 4-year mortality of 39% (adjusted HR 0.88; 95% CI 0.46–1.70). There was a significant interaction between MVRe and treatment arms, with a more favorable effect of MVRe on survival in patients undergoing CABG compared with those receiving CABG+SVR (adjusted interaction p-value=0.013). Similar results were observed when the small number of patients who had received MV replacement for moderate or severe MR at baseline were also included in our analyses (adjusted interaction p-value=0.008). Among those who did not undergo concomitant MV surgery despite having moderate or severe MR at baseline, the difference in all-cause mortality between the patients who received CABG only and those who underwent CABG+SVR was not significant (p=0.167).
Figure 1.
Impact of mitral valve repair on mortality in patients with moderate or severe mitral regurgitation (MR) at baseline randomized to CABG or CABG+SVR. CABG, coronary artery bypass grafting; SVR, surgical ventricular reconstruction.
Changes in severity of mitral regurgitation after mitral valve repair
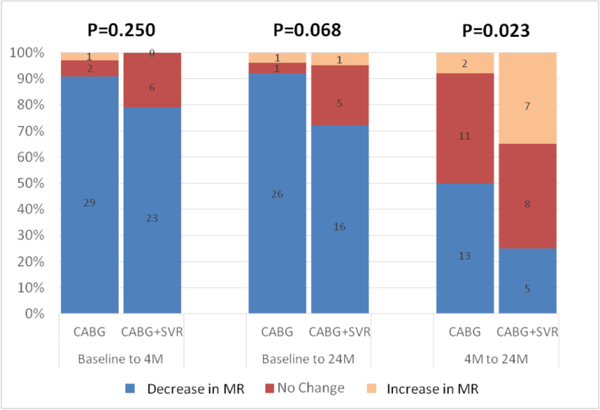
Among patients who underwent concomitant MVRe at the time of revascularization, baseline MR severity was similar between the group that received CABG+MVRe (44 patients) and those that underwent CABG+SVR+MVRe (48 patients) (mean MR grade 2.8±0.8 vs. 2.7±0.8, p=0.618). At 4-month follow-up, there was no difference in MR grade between the 2 groups (mean MR grade 1.1±0.9 vs. 1.1±0.8, p=0.841). In addition, both groups demonstrated a similar degree of reduction in MR from baseline to 4-month follow-up (mean reduction in MR grade 1.8±1.0 vs. 1.7±1.3, p=0.878). Changes in MR over time between these two groups of patients (at both 4 months and 24 months) are shown in Figure 2. Among patients with moderate or severe baseline MR, a proportionately greater number of patients who received CABG+MVRe demonstrated a decrease in MR between 4- and 24-month follow-up compared with those who underwent CABG+SVR+MVRe (50.0% versus 25.0%). In contrast, a larger percentage of patients who underwent CABG+SVR+MVRe showed an increase in MR between 4- and 24-month follow-up compared with those who received CABG+MVRe (35.0% versus 7.7%). The pattern of changes in MR over time was significantly different between the two groups (p=0.023).
Figure 2.
Comparison of changes in mitral regurgitation over time in patients with moderate or severe MR who underwent CABG+mitral valve repair versus CABG+SVR+mitral valve repair. CABG, coronary artery bypass grafting; MR, mitral regurgitation; SVR, surgical ventricular reconstruction; 4M, 4-month follow-up; 24M, 24-month follow-up.
Discussion
The current data indicate that in patients with ischemic cardiomyopathy and moderate or severe MR at baseline, the addition of MVRe to CABG appears to provide a survival benefit over CABG alone, and MVRe may have a more favorable effect on survival in patients undergoing CABG than those undergoing CABG+SVR. In addition, there was a greater reduction in MR severity among patients who received CABG+MVRe than those who underwent CABG+SVR+MVRe.
Several studies have reported a lack of survival advantage from combining MV surgery with CABG in patients with ischemic MR 17–21. In contrast, results from STICH Hypothesis 1 patients with moderate or severe MR suggest that the combination of CABG and MVRe improved survival when compared with CABG alone despite more non-fatal complications during the early postoperative period 10. An additional observation was a higher risk of perioperative deaths in patients treated with CABG alone in the setting of significant MR, highlighting the possible harm associated with the failure to address the MR at the time of CABG. Our results are consistent with those observed in the STICH Hypothesis 1 patients in that the addition of MVRe to CABG was associated with better long-term survival than CABG alone. It should be noted that other studies which revealed a lack of mortality benefit associated with the addition of MV surgery to CABG typically included patients with higher baseline LVEF, and the follow-up duration was shorter. The discrepancy between our results and those reported by others potentially suggests that the clinical impact of significant MR may be more important among patients with more severe LV dysfunction, who may benefit from surgical intervention for their MR at the time of CABG.
Interestingly, our analysis reveals a significant interaction between MVRe and treatment arms, as MVRe had a more favorable effect on survival in patients undergoing CABG than in those receiving CABG+SVR. These findings suggest that the addition of SVR to CABG may negate the anticipated survival benefit associated with MVRe and even reduce the long-term survival in these patients. This observation may be related in part to worsening of MR severity, sphericity index, and diastolic function after SVR 6,8,22.
A recent study has demonstrated that combined MVRe and SVR led to a greater reduction in LV end-systolic volume and plasma brain natriuretic peptide level when compared with MVRe alone in patients with ischemic cardiomyopathy and functional MR, but there was no difference in 4-year survival 23. The lack of survival benefit associated with MV surgery when combined with SVR, an observation shared by us and others, might be related to the recurrence or progression of MR during follow-up. This hypothesis is supported by our serial postoperative data demonstrating less regression of MR in patients receiving CABG+SVR+MVRe compared to CABG+MVRe, and with a significantly greater number of patients manifesting worsening of MR after surgery in the CABG+SVR+MVRe group.
Several other studies have examined the clinical and hemodynamic effects of SVR, with or without adjuvant MV surgery, in patients with ischemic cardiomyopathy. While SVR generally results in a reduction in LV volume and an improvement in LVEF, worsening of LV geometry and other hemodynamic parameters is not uncommon 5,7–9. One of the earliest studies of the hemodynamic effect of SVR in patients with LV aneurysm suggest that the presence of a restrictive LV diastolic filling pattern postoperatively is a strong predictor of mortality 24. In another study, among patients undergoing SVR with or without concurrent CABG and/or MV surgery, MR grade improved immediately after surgery but subsequently worsened after 6 months to 2 years 7. These changes correlated with an initial reduction in LV volume followed by a subsequent LV and mitral annulus dilatation. Thus, the initial reverse remodeling of the LV with SVR may not be sustainable. Similarly, prior studies have reported worsening of MR in 20–40% of patients after SVR 6,8. Our results are consistent with those reported by a recently published analysis, which has demonstrated an increase in the sphericity index among those who received CABG+SVR compared with those who underwent CABG alone in the Hypothesis 2 component of the STICH trial 22. Interestingly, severity of MR was found to have significantly improved only in those who underwent CABG, while worsening of LV filling pressures was seen only in patients who received CABG+SVR 22.
Limitations
Our analyses were limited by a relatively small number of patients with moderate or severe MR at baseline. In addition, while the decision to perform CABG or CABG+SVR was randomized, the decision to perform MV surgeries in STICH was not based on a prospective secondary randomization. In the current report, mortality rates of patients in different treatment groups were therefore compared after adjusting for propensity factors related to whether MVRe was performed to minimize the effects of confounding. It is reassuring that patients with moderate or severe MR in different surgical treatment groups were similar in number and had comparable baseline characteristics. In addition, in order to ensure that our results can be generalized to everyday practice, some of our analyses were repeated using data on severity of baseline MR reported by participating study sites. The differential impact of MV surgery on mortality rates of patients receiving CABG with or without SVR was again observed (Supplemental Figure 1).
While our data demonstrate differences in changes in MR severity over time between patients who underwent CABG+SVR+MVRe versus CABG+MVRe, this analysis should be interpreted with caution. Since a comparison of MR between preoperative and follow-up assessment is possible only in patients who survived to their follow-up echocardiographic examination, our analysis could have been influenced by survival bias. Nevertheless, this observed discrepancy in the changes in MR after surgery does offer a plausible explanation for the absence of anticipated benefit associated with MVRe when combined with SVR in patients with significant MR.
Conclusion
Among patients with moderate or severe MR in the setting of ischemic cardiomyopathy, MVRe in addition to CABG was associated with improved survival compared to CABG without MVRe, suggesting that CABG alone may be insufficient in these patients. MVRe appeared to have a more favorable effect on survival in patients undergoing CABG than those who received CABG+SVR. We speculate that the addition of SVR may attenuate the anticipated benefit of MVRe, and this observation may be related in part to a different evolution of MR severity after MVRe between patients undergoing CABG and those who underwent CABG+SVR.
Supplementary Material
Supplemental Figure 1. Impact of mitral valve surgery on patients with moderate or severe mitral regurgitation (MR) at baseline (based on severity of MR reported by participating study sites) who underwent CABG versus CABG+SVR. CABG, coronary artery bypass grafting; SVR, surgical ventricular reconstruction.
Supplemental Figure 2. Impact of mitral valve repair on cardiovascular mortality in patients with moderate or severe mitral regurgitation (MR) at baseline randomized to CABG or CABG+SVR. CABG, coronary artery bypass grafting; SVR, surgical ventricular reconstruction
Supplemental Figure 3. Comparison of changes in mitral regurgitation over time in patients with moderate or severe MR who underwent CABG versus CABG+mitral valve repair. CABG, coronary artery bypass grafting; MR, mitral regurgitation; MVR, mitral valve repair; 4M, 4-month follow-up; 24M, 24-month follow-up.
Acknowledgments
Disclosures statement
Sources of Funding
This work was supported by grants U01-HL69015, U01-HL69013, and R01-HL10583 from the National Heart, Lung, and Blood Institute/National Institutes of Health. The views expressed in this manuscript do not necessarily reflect those of the NHLBI or the NIH.
Disclosures
Paul A. Grayburn:
Research Grants (PI/Consultant): Abbott Vascular, Boston Scientific, Medtronic, Edwards Lifesciences, Tendyne, ValTech Cardio, Neochord – significant
Eric J. Velazquez:
Research Grants (PI/Consultant): NHLBI, Alnylam Pharmaceuticals, Pfizer, Amgen, Novartis – significant
Honoraria (Speaker): Expert Exchange – significant
Footnotes
All other authors:
None
Clinical Trial Registration – URL: http://www.clinicaltrials.gov. Unique identifier: NCT00023595.
References
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. [DOI] [PubMed] [Google Scholar]
- 2.Athanasuleas CL, Stanley AW Jr., Buckberg GD. Restoration of contractile function in the enlarged left ventricle by exclusion of remodeled akinetic anterior segment: surgical strategy, myocardial protection, and angiographic results. J Card Surg. 1998;13(6):418–428. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EJ, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. The Journal of thoracic and cardiovascular surgery. 2007;134(6):1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360(17):1705–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bove T, Van Belleghem Y, Vandenplas G, et al. Short-term systolic and diastolic ventricular performance after surgical ventricular restoration for dilated ischemic cardiomyopathy. Eur J Cardiothorac Surg. 2009;35(6):995–1003; discussion 1003. [DOI] [PubMed] [Google Scholar]
- 6.Di Donato M, Sabatier M, Dor V, et al. Effects of the Dor procedure on left ventricular dimension and shape and geometric correlates of mitral regurgitation one year after surgery. J Thorac Cardiovasc Surg. 2001;121(1):91–96. [DOI] [PubMed] [Google Scholar]
- 7.Menicanti L, Di Donato M. Surgical left ventricle reconstruction, pathophysiologic insights, results and expectation from the STICH trial. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2004;26 Suppl 1:S42–46; discussion S46–47. [PubMed] [Google Scholar]
- 8.Qin JX, Shiota T, McCarthy PM, et al. Importance of mitral valve repair associated with left ventricular reconstruction for patients with ischemic cardiomyopathy: a real-time three-dimensional echocardiographic study. Circulation. 2003;108 Suppl 1:II241–246. [DOI] [PubMed] [Google Scholar]
- 9.Ueno T, Sakata R, Iguro Y, Yamamoto H, Ueno M, Matsumoto K. Mid-term changes of left ventricular geometry and function after Dor, SAVE, and Overlapping procedures. Eur J Cardiothorac Surg. 2007;32(1):52–57. [DOI] [PubMed] [Google Scholar]
- 10.Deja MA, Grayburn PA, Sun B, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125(21):2639–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michler RE, Smith PK, Parides MK, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med. 2016;374(20):1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh JK, Pellikka PA, Panza JA, et al. Core lab analysis of baseline echocardiographic studies in the STICH trial and recommendation for use of echocardiography in future clinical trials. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2012;25(3):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39 e14. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 17.Kang DH, Kim MJ, Kang SJ, et al. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation. 2006;114(1 Suppl):I499–503. [DOI] [PubMed] [Google Scholar]
- 18.Smith PK, Puskas JD, Ascheim DD, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371(23):2178–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation. 2012;126(21):2502–2510. [DOI] [PubMed] [Google Scholar]
- 20.Castleberry AW, Williams JB, Daneshmand MA, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: a 20-year experience. Circulation. 2014;129(24):2547–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fattouch K, Sampognaro R, Speziale G, et al. Impact of moderate ischemic mitral regurgitation after isolated coronary artery bypass grafting. The Annals of thoracic surgery. 2010;90(4):1187–1194. [DOI] [PubMed] [Google Scholar]
- 22.Choi JO, Daly RC, Lin G, et al. Impact of surgical ventricular reconstruction on sphericity index in patients with ischemic cardiomyopathy: follow-up from the STICH trial. Eur J Heart Fail. 2015;17(4):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kainuma S, Taniguchi K, Toda K, et al. Restrictive mitral annuloplasty with or without surgical ventricular reconstruction in ischemic cardiomyopathy: impacts on neurohormonal activation, reverse left ventricular remodelling and survival. Eur J Heart Fail. 2014;16(2):189–200. [DOI] [PubMed] [Google Scholar]
- 24.Salati M, Paje A, Di Biasi P, Fundaro P, Cialfi A, Santoli C. Severe diastolic dysfunction after endoventriculoplasty. J Thorac Cardiovasc Surg. 1995;109(4):694–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Impact of mitral valve surgery on patients with moderate or severe mitral regurgitation (MR) at baseline (based on severity of MR reported by participating study sites) who underwent CABG versus CABG+SVR. CABG, coronary artery bypass grafting; SVR, surgical ventricular reconstruction.
Supplemental Figure 2. Impact of mitral valve repair on cardiovascular mortality in patients with moderate or severe mitral regurgitation (MR) at baseline randomized to CABG or CABG+SVR. CABG, coronary artery bypass grafting; SVR, surgical ventricular reconstruction
Supplemental Figure 3. Comparison of changes in mitral regurgitation over time in patients with moderate or severe MR who underwent CABG versus CABG+mitral valve repair. CABG, coronary artery bypass grafting; MR, mitral regurgitation; MVR, mitral valve repair; 4M, 4-month follow-up; 24M, 24-month follow-up.