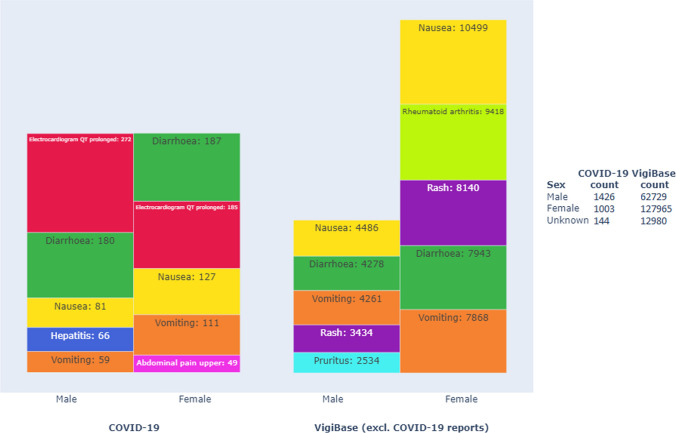
Fig. 3.
Top five reported adverse drug reactions (MedDRA® preferred terms), with number of reported instances, for the included drugs used in treating COVID-19 (left) vs. for the same drugs used in other indications (right), separated by sex. Reports with unknown sex and the MedDRA® preferred terms off-label use, intentional product use issue, and drug ineffective are excluded. The size of the box represents the proportions within the COVID-19 and VigiBase datasets, respectively. Reports may contain more than one reported preferred term. The legend shows the number of reports for each subset