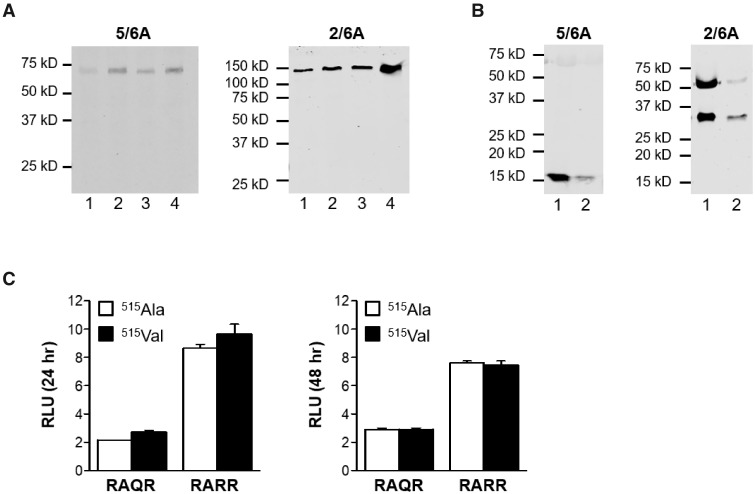
Figure 2.
Western blot analysis and bioactivity of anti-Müllerian hormone (AMH) proteins AMH-515Ala and AMH-515Val. Western analysis of HEK293 cells expressing the hAMH variants without (RAQR) or with (RARR) the optimized cleavage site were analyzed. The mature region-specific 5/6A antibody recognizes the AMH precursor protein (75 kD) and the cleaved C-terminal mature protein (∼15 kD). The pro-region-specific 2/6A antibody recognizes the dimeric AMH precursor protein (150 kD) and the cleaved N-terminal pro-region (∼57 kD). The relative molecular masses (kD) of the protein marker are indicated on the left. No difference was observed in processing of the AMH variants. Cell lysates (A) Lane 1: hAMH-RAQR-515Ala; lane 2: hAMH-RAQR-515Val; lane 3: hAMH-RARR-515Ala; lane 4: hAMH-RARR-515Val. Supernatants (B) Lane 1: hAMH-RARR-515Ala; lane 2: hAMH-RARR-515Val. (C) Analysis of AMH-515Ala and AMH-515Val bioactivity. KK1/AMHR2 cells were transiently transfected with the AMH-responsive BRE-Luc reporter plasmid together with the hAMH variants without (RAQR) or with (RARR) the optimized cleavage site. Luciferase was measured after 24 and 48 h incubation. No differences were observed in luciferase stimulation by both variants. Data are presented as relative luciferase units (RLU) expressed relative to the empty vector control. Data points are the mean ± SEM of triplicates of a representative experiment, which was repeated at least three times.