Abstract
STUDY QUESTION
Does follicular flushing increase the number of mature oocytes in monofollicular IVF?
SUMMARY ANSWER
Follicular flushing increases the number of mature oocytes in monofollicular IVF.
WHAT IS KNOWN ALREADY
Flushing increases neither the oocyte yield nor the pregnancy rate in polyfollicular IVF or in poor responder patients. In monofollicular IVF, the effect of flushing has so far been addressed by two studies: (i) a prospective study with minimal stimulation IVF demonstrated an increased oocyte yield, and (ii) a retrospective study with natural cycle (NC)-IVF showed an increased oocyte yield and an increased transfer rate.
STUDY DESIGN, SIZE, DURATION
Randomized controlled trial including 164 women who were randomized for either aspiration with or without flushing from 2016 to 2019.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Infertile women 18–42 years of age with an indication for IVF treatment at a university-based infertility unit. Women undergoing monofollicular IVF were randomized to either follicular aspiration only or follicular aspiration directly followed by five follicular flushes at a 1:1 ratio. The intervention was done without anaesthesia, using a gauge 19 single-lumen needle. Flushing volume was calculated (sphere formula) based on the size of the follicle.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 164 women were included; 81 were allocated to ‘aspiration only’ and 83 to additional ‘flushing’. Primary analysis was based on the intention-to-treat: oocyte yield, defined as the collected mature oocyte rate, was higher (n = 64/83, 77.1%) in the flushing group compared to the aspiration only group (n = 48/81, 59.3%, adjusted risk difference (RD): 18.2% (95% CI 3.9–31.7%), P-value = 0.02). In the flushing group, most oocytes were retrieved within the first three flushes (63/83, 75.8%). Fertilization rate was higher in the flushing group (n = 53/83, 63.9% vs n = 38/81, 46.9%; adjusted RD: 16.8% (96% CI 1.5–31.4%), P = 0.045). Transfer rate was also higher in the flushing group (n = 52/83, 62.7% vs n = 38/81, 46.9%; RD: 15.71 (95% CI 0.3–30.3%)), but the difference was not significant (P = 0.06). The clinical pregnancy rate n = 9/83 versus n = 9/81 (RD: −0.3% (95% CI −9.9% to 9.5%)) and live birth rate n = 7/83 versus n = 8/81 (RD: −1.5% (95% CI −10.4% to 7.1%)) were not significantly different between the flushing and the aspiration group. The median duration of the intervention was significantly longer with flushing (2.38 min; quartiles 2.0, 2.7) versus aspiration only (0.43 min; quartiles 0.3, 0.5) (P < 0.01). There was no significant difference in the mean (±SD) visual analogue scales pain score between the follicular flushing (3.4 ± 1.8) and the aspiration group (3.1 ± 1.89).
LIMITATIONS, REASONS FOR CAUTION
Blinding of the procedure was not possible.
WIDER IMPLICATIONS OF THE FINDINGS
Our study proved that flushing of single follicles in NC-IVF increases the oocyte yield. In contrast to polyfollicular IVF flushing seems to be beneficial in a monofollicular setting if the technique used in our study (single-lumen needle, 5 flushings with flushing volume adaptation) is applied.
STUDY FUNDING/COMPETING INTEREST(S)
The study was funded by the financial sources of the division and in part by a research grant provided by NMS Biomedical SA, Switzerland. The company did not have any roles in design or conduct of the study or in the preparation of the manuscript. The authors have no other conflicts of interest.
TRIAL REGISTRATION NUMBER
Clinicaltrials.gov NCT 02641808.
TRIAL REGISTRATION DATE
29 December 2015
DATE OF FIRST PATIENT’S ENROLMENT
22 August 2016
Keywords: follicular flushing, IVF, monofollicular IVF, single-lumen needle, oocyte yield, live birth rate
Introduction
Flushing of follicles was a routine procedure in IVF treatments at the beginning of ultrasound-guided follicular aspiration (Waterstone and Parsons, 1992). The purpose of the flushing procedure was to increase oocyte yield, possibly by improved detachment of the cumulus–oocyte complex (COC) from the follicular wall. In polyfollicular IVF, several studies showed that flushing increased neither oocyte yield nor pregnancy rate per cycle in patients with a normal response to gonadotropin stimulation (Levy et al., 2012; Roque et al., 2012; Georgiou et al., 2018).
However, in poor responder patients (Ferraretti et al., 2011) the benefit of flushing has been a subject of controversy. One randomized controlled trial (RCT) demonstrated that this approach did not improve oocyte yield but led to a 32% longer operation time (Haydardedeoglu et al., 2017). Two other RCTs were consistent with these results; neither found an increased oocyte yield by flushing the follicles (Mok-Lin et al., 2013; Von Horn et al., 2017). In contrast, two large retrospective studies revealed a higher number of oocytes if follicles were flushed (Souza et al., 2017; Xiao et al., 2018).
With regard to monofollicular IVF, few studies have addressed the efficacy of follicular flushing. It is obvious that even a small difference in the oocyte yield will have a significant impact on the efficacy of monofollicular IVF, as only one follicle is available for aspiration.
One prospective study demonstrated an increased oocyte yield (Méndez Lozano et al., 2007); this result was confirmed by a retrospective study in which three flushes almost doubled not only the number of aspirated oocytes but also the transfer rate in natural cycle-IVF (NC-IVF) (von Wolff et al., 2013).
To clarify if flushing is beneficial in monofollicular IVF, we performed a randomized study and evaluated not only the oocyte yield but also the oocyte maturity. In addition, we analysed the fertilization rate and the transfer rate in women who received either a single aspiration of the follicles or an aspiration followed by five follicular flushes. We then analysed the pain perceived and the time required for the flushing procedure. This study was intended to determine whether flushing could improve the success of monofollicular IVF.
Materials and methods
Study design and participants
We conducted an RCT of follicular flushing in women undergoing gonadotropin-free monofollicular IVF. Enrolment at our infertility clinic began in August 2016 and was completed in May 2019. Inclusion criteria were as follows: women aged from 18 to 42 years with the indication and the desire of gonadotropin-free monofollicular IVF and fertilization via ICSI. The women had to have a regular menstrual cycle (26–32 days) and transvaginal reachable ovaries for follicular aspiration. On the day of oocyte pick-up (OPU), we only included women with a single follicle of ≥16 mm in diameter. Exclusion criteria were women with more than two previous embryo transfers without pregnancy, an LH surge on the trigger day, or previous enrolment in the current study. We offered gonadotropin-free monofollicular IVF to all women with a regular menstrual cycle.
Investigation
Women were informed about the study when being counselled about IVF treatment. Monofollicular IVF was defined as IVF therapies within the natural menstrual cycle in which women injected only 5000 units of urinary human chorionic gonadotropin to trigger ovulation. In addition, women were allowed to be treated additionally with doses of CC (clomiphene citrate 25 mg/day from Day 6 until induction of ovulation) to reduce the risk of premature ovulation as described elsewhere (von Wolff et al., 2014). We performed one follicular monitoring, individually scheduled depending on the women’s cycle length, before ovulation induction to assess follicular maturity. Only monofollicular cycles were included in the study. Ovulation was induced 36 h before OPU. We enrolled participants after all eligibility criteria were verified and informed consent was signed before OPU.
On the day of OPU, after confirmation of the presence a follicle (≥16 mm) by transvaginal ultrasound scan, we randomized the patients real-time online who could be included in the study, to either the follicular flushing or the aspiration only study arm. Patients, investigators and embryologists could not be blinded for the procedure. Follicles were aspirated with an aspiration pressure of 220 mmHg to achieve a flow rate of 20–25 ml/min, which is the value suggested for oocyte retrieval with minimal damage to the COC and zona pellucida, following the manufacturer’s suggestion. It was done without anaesthesia or analgesia, using gauge (G) 19 single-lumen needles (NMS Biomedical SA, Praroman, Switzerland) (von Wolff et al., 2014). In the aspiration only group, the needle was retracted after emptying the follicle, whereas in the follicular flushing group, the follicle was aspirated and the needle was left inside the follicle to flush the follicles 5 times with a flushing medium containing heparin (SynVitro® Flush, Origio, Berlin, Germany). Flushing volume was calculated (sphere formula) based on the size of the follicle. The needle was rinsed at the end of the aspirations. We defined ‘oocyte yield’ as the proportion of mature oocytes retrieved per randomized women. Fertilization was achieved by standard ICSI.
The oocyte yield, the fertilization rate (proportion of pronuclear stages per women), and the transfer rate per women were analysed for both groups. Furthermore, embryo quality was assessed by ASEBIR (Asociación para el Estudio de la Biología de la Reproducción) classification (De Los Santos et al., 2014), based on the number of cells, the heterogeneity of the cells and the fragmentation rate of the embryo. Embryos were transferred at cleavage stage (Day 2 or 3 after OPU) under ultrasound guidance.
Outcomes
The primary outcome was the probability of finding a mature (metaphase II (MII)) oocyte per cycle of aspiration. Secondary outcomes were the proportion of ‘any’ oocyte retrieved, the number of flushes needed to retrieve the oocyte (flushing group only), the fertilization rate, embryo quality 48 h after fertilization, transfer rate and implantation and clinical pregnancy rates. Post hoc exploratory analyses included clinical pregnancy and live birth rates in women with an embryo transfer. Safety outcomes included intensity of pain during the intervention, evaluated by visual analogue scales (VAS) (Carlsson et al., 1983) filled out immediately after the intervention and the time needed for the intervention from the insertion of the needle to retraction. All data were collected and stored in a REDCap database (REDCap 8.5.19 Vanderbilt University, Nashville, USA) hosted by the Clinical Trial Unit (CTU) of the University of Bern.
Sample size
The sample size calculation was based on the primary outcome. We assumed a proportion of mature oocytes of 80% in the flushing group, compared to 60% in the aspiration only group deduced from our previous retrospective study (von Wolff et al., 2013). Based on a chi-squared test, 164 patients (82 per group) were required in order to detect a risk difference (RD) of 20% between the two groups in the final analysis, with a power of 80% at a two-sided, adjusted alpha-level of 0.049.
Randomization
Patients were allocated to the follicular flushing group and the aspiration only group in a 1:1 ratio. We used block randomization with block sizes of two, four and six, stratified according to age (≤35 vs >35 years) and stimulation scheme (NC without vs with 25 mg CC). We used Stata (Stata Corporation, College Station, TX, USA) to generate randomization lists. An independent data manager (CTU Bern) integrated these lists into the data entry and management system (REDCap), in order to ensure concealment of allocation. The investigators performed online randomization immediately before the intervention.
Statistical analysis
An interim analysis of the primary outcome after the inclusion of the first 82 patients was conducted in order to stop the trial earlier than planned if there was convincing evidence of benefit. In order to control the overall Type I error rate, the O’Brien–Fleming group sequential design was used (O’Brien and Fleming, 1979), testing the primary hypothesis at a significance level of 0.0031 and 0.049 at the interim and the final analysis, respectively. As the significance level of 0.0031 defined in the protocol for testing the primary hypothesis was not reached at the interim analysis, the trial was continued until the end.
The primary analysis was an intention-to-treat (ITT) analysis. In a per-protocol analysis, only patients that adhered to the protocol were evaluated.
For the primary outcome (proportion of mature oocytes) as well as binary secondary outcomes (fertilization, transfer, implantation, and clinical pregnancy rate), a stratified Mantel–Haenszel RD between the two groups with a 95% CI was calculated. The groups were compared using a Cochran–Mantel–Haenszel test controlled for the stratification factors (age and stimulation scheme) used in the randomization.
For continuous secondary outcomes (embryo score, pain measured on a VAS scale and duration of intervention), the mean with SD and the median interquartile range were calculated. Groups were compared using linear regression adjusting for stratification factors. Non-normal data were normalized before the analysis using log- or square root-transformation. Subgroup analysis was performed according to age (≤35 vs >35 years) and stimulation protocol (NC vs modified NC with CC). In the flushing group, the association of the number of flushes with the embryo score was assessed in an ordered logistic regression. Analysis was specified in the study protocol and the statistical analysis plan; the analysis was performed by an independent statistician (CTU Bern) using R version 3.6.0.
Trial registration and approval
This study was reviewed and approved by the Cantonal Ethical Committee of Berne, Switzerland (KEK-BE 2015-00150). The trial was registered at Clinicaltrials.gov (NCT 02641808). The contents of the paper follow the CONSORT guidelines (Schulz et al., 2010). There were no changes in eligibility criteria, methods or measured outcomes after the beginning of the trial.
Details of ethical approval
Kantonale Ethikkomission Bern (KEK): 2015-00150 (Project ID).
Date of approval: 15 June 2016.
Results
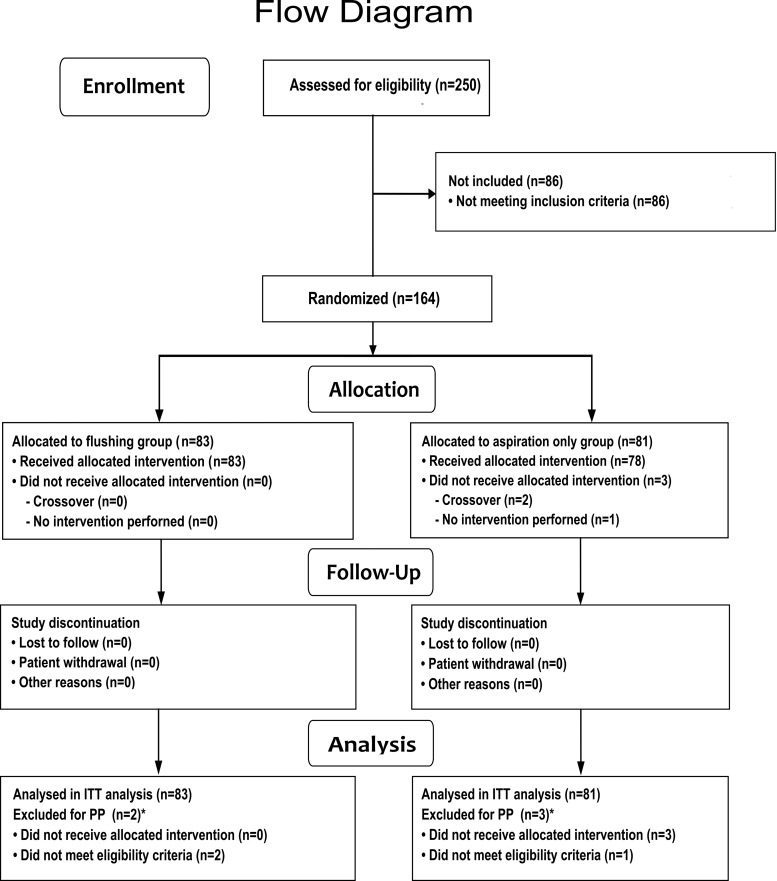
A total of 164 women were included and randomized, 83 in the follicular flushing group and 81 in the aspiration only group and analysed according to the ITT principle (Fig. 1). Due to anatomical reasons (ovary too high up in the abdomen), one aspiration was not performed at all. Two interventions were not performed according to the result of the randomization (cross-over) by mistake of the investigator.
Figure 1.
Flow chart of enrolment, allocation, follow-up and analysis of the study population. The asterisks indicate that multiple reasons may apply. ITT, intention-to-treat; PP, per-protocol.
Table I shows demographic data, parameters of reproductive history and follicular monitoring of the follicular flushing and the aspiration only group. There were no differences in age and in BMI between the two groups.
Table I.
Demographic and treatment characteristics.
| Overall n = 164 | Flushing group n = 83 | Aspiration only group n = 81 | |
|---|---|---|---|
| Age (years) | 35.0 [33.0; 38.0] | 35.0 [32.5; 38.0] | 35.0 [33.0; 38.0] |
| 18–35 | 90 (54.9) | 46 (55) | 44 (54) |
| 36–42 | 74 (45.1) | 37 (45) | 37 (46) |
| BMI (kg/m2) | 21.7 [20.2; 23.8] n = 163 | 21.7 [20.4; 23.8] | 21.6 [20.0; 23.8] n = 80 |
| Stimulation protocol | |||
| NC | 126 (76.8) | 64 (77) | 62 (76) |
| With CC | 38 (23.2) | 19 (23) | 19 (24) |
| Previous pregnancies | |||
| Nulligravid | 99 (60.4) | 50 (60) | 49 (60) |
| Gravid | 65 (39.6) | 33 (40) | 32 (40) |
| Previous embryo transfer(s) | |||
| 0 | 99 (60.4) | 50 (60) | 49 (60) |
| 1 or 2 | 65 (39.6) | 33 (40) | 32 (40) |
| Anti-Mullerian hormone (pmol/l) | 14.1 [6.1; 27.3] | 14.8 [5.8; 27.0] | 14.0 [6.9; 26.9] |
| <7.8 | 47 (28.7) | 26 (31) | 21 (26) |
| Reason for infertility (indication for IVF) | |||
| Male factor | 106 (64.6) | 49 (59) | 57 (70) |
| Tube factor | 22 (13.4) | 13 (15) | 9 (11) |
| Idiopathic | 24 (14.6) | 13 (15) | 11 (13) |
| Endometriosis II–IV | 7 (4.4) | 5 (6) | 2 (3) |
| Other | 5 (3.0) | 3 (4) | 2 (3) |
| Follicular monitoring | |||
| Main follicule size (mm) | 18.0 [17.0; 20.0] | 18.0 [17.0; 20.0] | 18.0 [17.0; 20.0] |
| Right side | 86 (52) | 47 (57) | 39 (48) |
| Left side | 78 (48) | 36 (43) | 42 (52) |
| Endometrium thickness (mm) | 7.1 [6.2; 8.6] | 7.1 [6.3; 8.6] | 7.1 [6.1; 8.3] |
| Estradiol level (pmol/l) | 556 [411; 789] | 524 [415; 780] | 580 [409; 790] |
| LH level (mU/l) | 7.7 [5.8; 10] | 7.7 [5.9; 9.9] | 7.8 [5.8; 10] |
Values are represented as number n (%) or median [quartiles].
CC, clomiphene citrate; NC, natural cycle.
Oocyte yield, transfer and pregnancy rates
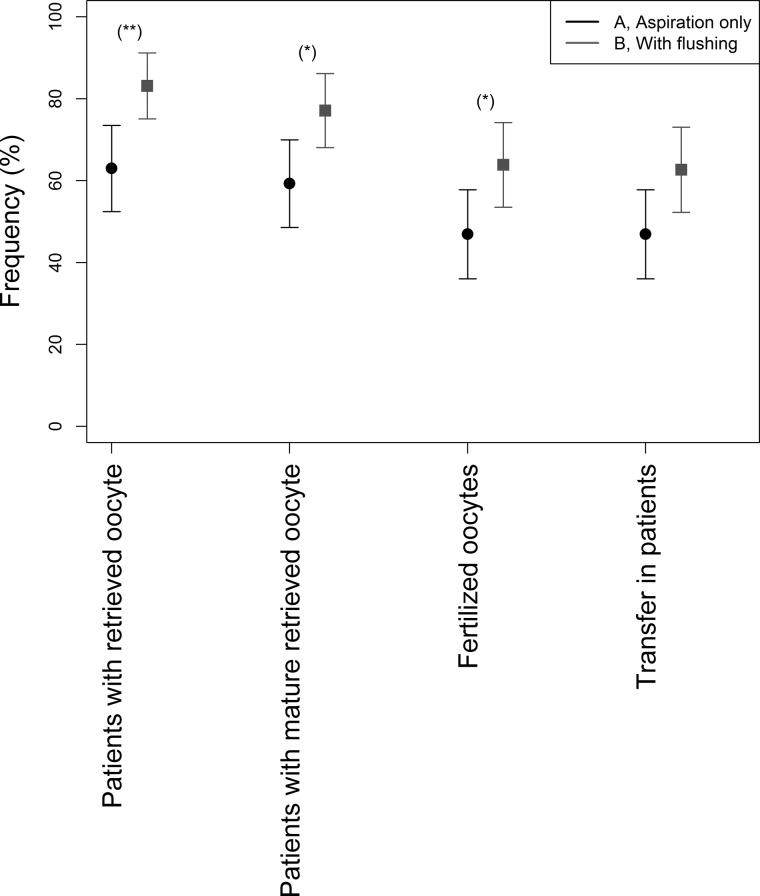
The total oocyte retrieval rate was higher (69/83, 83%) after follicular flushing versus aspiration only (51/81, 63%), RD 20.6%; 95% CI 7.06–33.2%; P < 0.01.
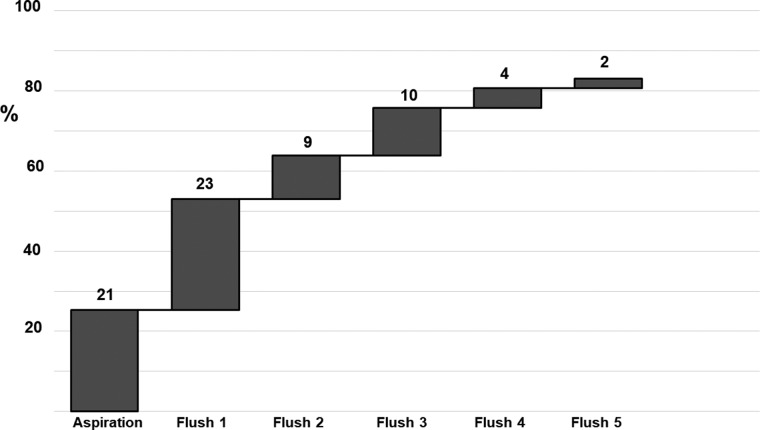
In the flushing group, 21 oocytes (25%) were retrieved at the initial aspiration, 23 (28%) in the first, 9 (11%) in the second, 10 (13%) in the third, 4 (5%) in the fourth and 2 (2%) in the fifth flushing step. The total oocyte retrieval increased from 25% after initial aspiration to 76% after the third flush and to 83% after the fifth flush (Fig. 2).
Figure 2.
Retrieval of oocytes per flush within the 5-fold flushed group (n = 83) in monofollicular IVF treatment.
The unadjusted RD in mature oocyte yield (MII oocytes) was 17.9% (95% CI 3.8–31.9%), based on 64/83, 77% with flushing versus 48/81, 59% with aspiration only. The adjusted RD 18.2% (95% CI 3.86–31.68%), P = 0.02. For fertilized embryos (64% vs 47%, RD 16.9%, 95% CI 1.51–31.46%, P < 0.05), the proportion was significantly higher in the follicular flushing group than in the aspiration only group (Table II and Fig. 3). There were two (2.4%) empty COC with a damaged zona in the flushing and one (1.2%) in the aspiration only group.
Table II.
Outcomes of the ITT and the PP analysis.
| Flushing group | Aspiration only group | Mantel–Haenzel risk difference (% [95% CI]) † | P-value | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| ITT analysis | n = 83 | n = 81 | ||
| Retrieved oocytes | 69 (83) | 51 (63) | 20.6 [7.1, 33.2] | 0.005 |
| Mature oocytes (MII) | 64 (77) | 48 (59) | 18.2 [3.9, 31.7] | 0.02 |
| Fertilization | 53 (64) | 38 (47) | 16.9 [1.5, 31.5] | 0.05 |
| Embryo transfers (transfer rate) | 52 (63) | 38 (47) | 15.7 [0.3, 30.4] | 0.06 |
| Implantation rate | 12 (15) | 10 (12) | 1.9 [−8.4, 12.1] | 0.89 |
| Clinical pregnancies/cycle | 9 (11) | 9 (11) | −0.5 [−9.9, 9.0] | 0.93 |
| Live birth/cycle** | 7 (8) | 8 (10) | −1.66 [−10.4, 7.1] | 0.72 |
| n = 52 | n = 38 | |||
| Clinical pregnancies/transfer* | 9 (17) | 9 (24) | −7.9 [−24.5, 8.8] | 0.51 |
| Live birth/transfer* | 7 (14) | 8 (21) | −9.1 [−24.7, 6.5] | 0.38 |
| PP analysis | n = 81 | n = 78 | ||
| Retrieved oocytes | 67 (83) | 49 (63) | 20.7 [7, 33.4] | 0.006 |
| Mature oocytes (MII) | 63 (78) | 46 (59) | 19.4 [4.8, 33] | 0.015 |
| Fertilization | 52 (64) | 36 (46) | 18.2 [2.6, 32.9] | 0.033 |
| Embryo transfers (transfer rate) | 51 (63) | 36 (46) | 17 [1.3, 31.8] | 0.048 |
| Implantation rate | 12 (15) | 10 (13) | 2.1 [−8.6, 12.6] | 0.88 |
| Clinical pregnancies/cycle | 9 (11) | 9 (12) | −0.4 [−10.2, 9.4] | 0.94 |
| Live birth/cycle** | 7 (9) | 8 (10) | −1.6 [−10.6, 7.4] | 0.70 |
| n = 51 | n = 36 | |||
| Clinical pregnancies/transfer* | 9 (18) | 9 (25) | −8.4 [−25.9, 9.0] | 0.49 |
| Live birth/transfer* | 7 (14) | 8 (22) | −9.5 [−25.9, 6.8] | 0.38 |
ITT, intention-to-treat; MII, metaphase II; PP, per-protocol.
Implantation rate per transfer is based on the subset analysis of women having had an embryo transfer.
Live birth rate per initiated cycle is part of the post hoc analysis.
With flushing—aspiration only, stratified for age and stimulation protocol.
Figure 3.
Outcome of monofollicular IVF cycles with follicular flushing (n = 83) versus aspiration only (n = 81). The treatment steps shown are follicles aspirated, oocytes retrieved, mature oocytes, oocytes fertilized and embryos transferred. Asterisks indicate significant differences between the groups.
The implantation rate was similar between the two groups (15% vs 12%, RD 1.92%, 95% CI −8.39% to 12.11%, P = 0.89) (Table II).
Clinical pregnancy rate per transfer did not differ between the follicular flushing group (9/52, 17.3%) and the aspiration group (9/38, 23.7%, RD −7.87% (95% CI −24.51–8.8%) P = 0.51). Finally, there were seven live births and two miscarriages in the flushing group versus eight live births and one miscarriage in the aspiration group.
Embryo quality
The proportion of embryos with a given embryo score were A (40.4%), B (23%), C (21.2%) and D (15.4%) in the follicular flushing group and A (18.4%), B (47.4%), C (21%) and D (13.2%) in the aspiration group. The median embryo score was not different (ASEBIR B) in the two groups (P = 0.33). In the flushing group, there was no significant association between the number of flushes and the quality of the embryo, as assessed by logistic regression analysis (odds ratio 1.39, 95% CI 0.93–2.11, P = 0.11) (Supplementary Fig. S1).
Secondary analysis
In a per-protocol analysis, two patients in the follicular flushing group and three patients in the aspiration only group were excluded (Fig. 1). Differences in primary and secondary outcomes were similar to the results of the ITT analysis (Table II).
In a subgroup analysis, RDs in retrieved mature oocytes were similar in women aged 18–35 and 36–42 years as well as in IVF cycles with and without CC (Supplementary Fig. S2).
Safety outcomes
The median duration of the intervention was significantly longer after follicular flushing (2.38 min; interquartile range (IQR) 2.00, 2.76) versus aspiration only (0.43 min; IQR 0.37, 0.54) (P < 0.01). There was no significant difference in the mean VAS pain score between the follicular flushing (3.41 ± 1.81) and the aspiration group (3.12 ± 1.83) (P = 0.31). In both groups, there was no clinically relevant bleeding, peritoneal infection or injury to pelvic organs.
Discussion
This is the first RCT to show that in monofollicular IVF follicular flushing results in a higher yield of mature oocytes and higher fertilization rate compared to aspiration only. In the follicular flushing group, three-quarters of the oocytes were retrieved within the first three flushes; the number of flushes performed did not affect the maturity and the quality of the embryos. Although the duration of the intervention was in median significantly longer in the follicular flushing group, the pain perceived was not statistically significantly different between the two groups.
This study strongly suggests that follicular flushing is beneficial in monofollicular IVF.
The strength of the study is the appropriate study design with power calculation-based inclusion of a clearly defined population.
The limitation of the study is the lack of blinding due to the study design, which can cause potential bias.
Flushing of follicles can be performed in three groups of IVF therapies, which all can be assumed different regarding flushing of the follicles due to the biology of the follicles but also due to the flushing technique applied. Therefore, the results of each group cannot be transferred from one to the other.
In the first group, the conventional gonadotropin IVF with a polyfollicular response, flushing increases neither oocyte yield nor pregnancy rate (Levy et al., 2012; Roque et al., 2012; Georgiou et al., 2018). The elaborated technique of oocyte retrieval in polyfollicular ovaries is mainly done under anaesthesia or sedation and in an operation theatre. The setting of the procedure is therefore completely different from monofollicular IVF, which is performed without any anaesthesia and sedation and, as in our centre, often outside the operation theatre.
In the second group, conventional gonadotropin IVF with low response such as in poor responders, most but not all studies also reveal that flushing of the follicles does not increase oocyte yield. However, some retrospective studies demonstrate an increased oocyte yield. Five studies have investigated follicular flushing in poor responders.
First, an RCT (Mok-Lin et al., 2013) performed with a single-lumen needle for direct aspiration and a double-lumen needle for the flushing group displayed a lower embryo transfer and implantation rate for the flushed oocytes. Furthermore, the study showed a potentially lower pregnancy and live birth rate. The author’s hypothesis for this finding is inter alia the increased intrafollicular pressure when the follicles were flushed at retrieval. Although neither in their study nor in our results, there was significant evidence of fractured zona in the flushing group (two oocytes vs one oocyte in the aspiration only group). Furthermore, granulosa cell function in luteal phase is not affected through the follicular flushing process in NC, as shown previously (von Wolff et al., 2017). The reason for a higher pregnancy rate in the Mok-Lin study after only aspiration remains unclear. Anyway, their study is not comparable to our trial because of differences in stimulation scheme (high-dose gonadotropin) and population (poor responder patients).
Second, a trial (Von Horn et al., 2017) where a double-lumen versus a single-lumen needle was tested, the effect of flushing showed even a trend for lower oocyte yield. The researchers used not only a partly double-lumen needle but also a semi-automatic flushing system, which exerts a much lower flushing pressure. The different equipment might be the reason for the different results of their study.
The third study, another RCT (Haydardedeoglu et al., 2017) in poor responders, showed that flushing did not improve oocyte yield and entailed a 32% increase in operative procedure time.
Later, two large, although retrospective, studies postulated an advantage of follicular flushes in poor responder patients (Souza et al., 2017; Xiao et al., 2018). They attributed their difference to a higher number of flushes (up to nine) and the reduced intra-follicular pressure caused by cautious intrafollicular injection.
Our study belongs to the third group, the monofollicular IVF. Our study strongly suggests that flushing is beneficial in monofollicular ovaries. It increases both the oocyte yield and the transfer rate. This is in line with previous studies, which suggest but did not yet prove an increased oocyte yield. We demonstrated in a previous retrospective study (von Wolff et al., 2013) that flushing increased the oocyte yield from 44.5% to 80.5%. Oocyte yield per aspiration was 44.5% in the aspirate, 20.7% in the first flush, 10.4% in the second flush and 4.3% in the third flush. Méndez Lozano et al. (2007) performed an aspiration without flushing in 79 women and with triple flushing in 47 women. They were stimulated with gonadotropins and controlled with GnRH antagonists in a semi-NC-IVF. The percentage of patients with a good embryo was 28.8% in the group without flushing and 37.8% in the group with flushing; however, the effect of the individual flushes was not analysed. Because there was a significant impact of follicular flushing on the number of oocytes retrieved and the number of MII oocytes, but not on the number of embryos transferred or the pregnancy rate, our study does not allow the conclusion that flushing also increases pregnancy rate.
The findings of all these studies indicate that the efficacy of the flushing procedure appears to depend on the number of follicles: the fewer follicles aspirated, the higher the efficacy of flushing. This raises the question of the reasons for these differences.
It can be assumed that the aspiration of polyfollicular and of monofollicular ovaries is physiologically and technically different. Polyfollicular ovaries consist of follicles of different sizes, including both large mature follicles and small immature follicles. In monofollicular ovaries, most follicles are large and mature. As detachment of the COC requires a certain degree of follicular maturity, it can be assumed that even heavy flushing might be insufficient to detach it.
Furthermore, the time-consuming procedure of the flushing of the follicles might also have an effect. The flushing of the follicles took at least 90 s longer than the aspiration of the follicles. Accordingly, thorough flushing of 10 follicles with a technique performed as in our study would require a 15 min longer aspiration time, and the flushing of 20 follicles would require a 30 min longer aspiration time. As this is impractical, it can be assumed that first, flushing of polyfollicular ovaries will be much less intensive, and second, such a long flushing time might result in adverse effects, which will decrease the efficacy of the flushing procedure. Accumulation of blood inside the aspirated follicles and the ovaries during the process of aspiration, for instance, might reduce the efficacy of the aspiration and the flushing.
The differences of the efficacy of flushing in poly- and monofollicular ovaries might also be due to differences in the technical equipment used for the aspiration. Polyfollicular ovaries are usually aspirated with thicker aspiration needles with a diameter of approximately 17 G to accelerate the aspiration procedure. We used a much thinner single-lumen needle, with a diameter of 19 G, to reduce the pain. One can speculate that the different diameters result in different flows of the flushing medium, due to the higher aspiration and flushing pressures in thin needles.
It is recognized that in the analysis of the oocyte yield after follicular flushing, it is always possible that the oocytes remain in the aspiration needle during the aspiration and are washed back into the follicle during flushing. Oocytes attributed to retrieval after flushing could adhere to the aspiration tubing and were therefore actually retrieved from aspiration alone, although credited to follicular flushing. To correct for that, a clearing of the dead space volume (0.9 ml) is mandatory. We therefore aimed to assess the oocyte yield by employing a direct comparison between follicular flushing versus aspiration only, where the needle is drawn back immediately. A follicle of 16 mm in diameter (volume of 2 ml) is rinsed five times with 2 ml flushing media each time. Accordingly, the chance that the oocyte is caught in the needle is around 50% per aspiration or flushing step. In a follicle with a diameter of 20 mm, corresponding to a volume of 4 ml and a flushing volume of 4 ml, the risk is 25% per aspiration/flushing step. However, several flushing steps can overcome this disadvantage.
Therefore, the proportion of ‘directly aspirated’ oocytes in the flushing group is not comparable to the ‘aspiration only’ oocytes.
We also studied how many flushing procedures are required. Three flushes led to an oocyte yield of 75.8%. Two further aspirations increased the success rate to 83.1%. Therefore, as two more flushes require only a minimal increase in aspiration time and as flushing is less painful than aspiration only, five flushes might be advantageous.
As several flushes might lead to an increased proportion of immature or dysfunctional oocytes, we also analysed the proportion of mature MII oocytes and the fertilization rate. Both were higher in the flushing group. We extended our study and analysed embryo quality to obtain more information regarding the oocyte competence. As the embryo quality was also not negatively affected, it seems that flushing does not negatively affect oocyte quality.
Conclusion
In conclusion, follicular flushing is beneficial in monofollicular IVF as it leads to a significantly higher oocyte yield and higher fertilization rate without affecting embryo quality. Our study proved that flushing of monofollicular ovaries via a single-lumen needle increases the efficacy of the aspiration. However, further studies are needed to prove that flushing also results in higher pregnancy and live birth rates.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Markus Eisenhut, Bruno Laborde and Laurence Richoz for technical support during the trial intervention.
Authors’ roles
A.S.K.S., I.C., A.L. and M.v.W. conceptualized and designed the study. A.S.K.S., A.F., A.W., S.W., V.R.M. and M.v.W. randomized the patients and collected the data. A.S.K.S., M.v.W. and A.L. conceptualized and M.R. and A.L. performed the statistical analysis. B.L. supervised the performance of the study. All authors contributed substantially to the interpretation of the findings. I.C. and A.S.K.S. wrote the first draft of the manuscript. All authors contributed to redrafting of the manuscript. A.S.K.S., B.L. and M.v.W. revised the article critically for important intellectual content. All authors approved the final submitted version.
Funding
The study was funded by the financial sources of the division and in part by a research grant provided by NMS Biomedical SA, Switzerland. The company did not have any rights regarding the design, conduct, analysis and publication of the study.
Conflict of interest
The authors report no conflict of interest.
References
- Carlsson AM. Assessment of chronic pain: I. Aspects of the reliability and validity of the visual analogue scale. pain1983;16:87–101. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011;26:1616–1624. [DOI] [PubMed] [Google Scholar]
- Georgiou EX, Melo P, Brown J, Granne IE.. Follicular flushing during oocyte retrieval in assisted reproductive techniques. Cochrane Database Syst Rev 2018;4:CD004634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydardedeoglu B, Gjemalaj F, Aytac P, Kilicdag E.. Direct aspiration versus follicular flushing in poor responders undergoing intracytoplasmic sperm injection: a randomised controlled trial. BJOG 2017;124:1190–1196. [DOI] [PubMed] [Google Scholar]
- Levy G, Hill MJ, Ramirez CI, Correa L, Ryan ME,, DeCherney AH, Levens ED, Whitcomb BW.. The use of follicle flushing during oocyte retrieval in assisted reproductive technologies: a systematic review and meta-analysis. Hum Reprod 2012;27:2373–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Santos MJ, Arroyo G, Busquet A, Calderón G, Cuadros J, Hurtado De Mendoza MV, Moragas M, Herrer R, Ortiz A, Pons C, et al. A multicenter prospective study to assess the effect of early cleavage on embryo quality, implantation, and live-birth rate. Fertil Steril 2014;101:981–987. [DOI] [PubMed] [Google Scholar]
- Méndez Lozano DH, Fanchin R, Chevalier N, Feyereisen E, Hesters L, Frydman N, Frydman R.. [The follicular flushing duplicate the pregnancy rate on semi natural cycle IVF]. J Gynecol Obstet Biol Reprod (Paris) 2007;36:36–41. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, Brauer AA, Schattman G, Zaninovic N, Rosenwaks Z, Spandorfer S.. Follicular flushing and in vitro fertilization outcomes in the poorest responders: a randomized controlled trial. Hum Reprod 2013;28:2990–2995. [DOI] [PubMed] [Google Scholar]
- O’Brien PC, Fleming TR.. A multiple testing procedure for clinical trials. Biometrics 1979;35:549. [PubMed] [Google Scholar]
- Roque M, Sampaio M, Geber S.. Follicular flushing during oocyte retrieval: a systematic review and meta-analysis. J Assist Reprod Genet 2012;29:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Altman D, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother 2010;1:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza ALM, Sampaio M, Noronha GB, Coster L GR, De Oliveira RSG, Geber S.. Effect of follicular flushing on reproductive outcomes in patients with poor ovarian response undergoing assisted reproductive technology. J Assist Reprod Genet 2017;34:1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterstone JJ, Parsons JH.. A prospective study to investigate the value of flushing follicles during transvaginal ultrasound-directed follicle aspiration. Fertil Steril 1992;57:221–223. [DOI] [PubMed] [Google Scholar]
- Von Horn K, Depenbusch M, Schultze-Mosgau A, Griesinger G.. Randomized, open trial comparing a modified double-lumen needle follicular flushing system with a single-lumen aspiration needle in IVF patients with poor ovarian response. Hum Reprod 2017;32:832–835. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Hua Y-ZZ, Santi A, Ocon E, Weiss B.. Follicle flushing in monofollicular in vitro fertilization almost doubles the number of transferable embryos. Acta Obstet Gynecol Scand 2013;92:346–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wolff M, Kohl Schwartz A, Stute P, Fäh M, Otti G, Schürch R, Rohner S.. Follicular flushing in natural cycle IVF does not affect the luteal phase—a prospective controlled study. Reprod Biomed Online 2017;35:37–41. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Nitzschke M, Stute P, Bitterlich N, Rohner S.. Low-dosage clomiphene reduces premature ovulation rates and increases transfer rates in natural-cycle IVF. Reprod Biomed Online 2014;29:209–215. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Wang Y, Wang M, Liu K.. Follicular flushing increases the number of oocytes retrieved in poor ovarian responders undergoing in vitro fertilization: a retrospective cohort study. BMC Womens Health 2018;18:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.