Fig. 1. Characterization of IF-PTI and PTI·H2O materials.
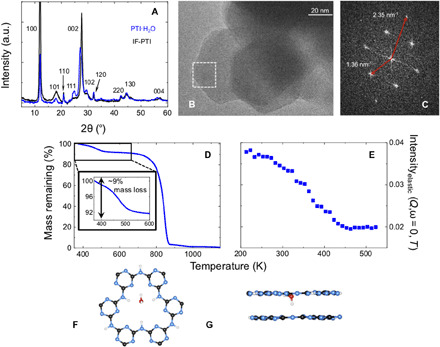
(A) Powder XRD patterns for IF-PTI and PTI·H2O. a.u., arbitrary units. (B) TEM image of hexagonal crystallites of IF-PTI showing lattice planes. (C) Fourier-transformed pattern showing characteristic diffraction spots of [110] (inner ring of spots in a hexagonal pattern) and [100] in-plane reflections of the PTI layers corresponding to real-space interatomic separations of 4.25 and 7.35 Å, respectively. (D) Thermogravimetric analysis (TGA) data for PTI·H2O between 300 and 1200 K. The initial reduction in mass of ~9% between 300 and 500 K is due to deintercalation of one H2O molecule per C6N9H3 formula unit of the layered PTI structure (19). The larger mass loss observed above 800 K is due to thermal decomposition of the IF-PTI carbon nitride phase. (E) Variation in elastic neutron scattering intensity observed during heating PTI·H2O between 200 and 530 K. (F and G) Top and side views of the C12N12H3 intralayer pores showing the equilibrium location of H2O molecules determined by DFT calculations.
