Abstract
Introduction
Both levetiracetam (LEV) and brivaracetam (BRV) eliminate the electroencephalogram photoparoxysmal response (PPR) in the human phase IIa photosensitivity model of epilepsy. The physiochemical properties of BRV differ from those of LEV, having higher potency and lipophilicity plus 10- to 15-fold greater affinity for synaptic vesicle glycoprotein 2A.
Objective
We compared the rapidity of the effects of both drugs in the central nervous system (CNS) of patients with photosensitive epilepsy using time to PPR elimination post-intravenous infusion as a pharmacodynamic endpoint.
Methods
Using a randomized, double-blind, two-period, balanced, crossover design, we tested patients with photosensitive epilepsy with equipotent milligram doses of intravenous LEV 1500 mg versus BRV 100 mg post-15-min intravenous infusion (part 1) and post-5-min intravenous infusion (part 2, same doses). Eight patients per part were deemed sufficient with 80% power to determine a 70% reduction for intravenous BRV:LEV intrapatient time ratio to PPR elimination, with a 0.05 two-sided significance level. Plasma antiseizure medicine concentrations were measured using liquid chromatography/mass spectrometry.
Results
Nine patients [six women; mean age 27.8 years (range 18–42)] completed the study; seven of these participated in both parts 1 and 2. In 31 of 32 instances, patients experienced PPR elimination. In mixed-effects model time analysis, BRV eliminated PPRs more quickly than did LEV (median 2 vs. 7.5 min, respectively). However, no statistically significant difference in BRV:LEV time ratio to PPR elimination was observed for two of our multiple primary outcomes: for the 15-min infusion alone (p = 0.22) or the 5-min infusion alone (p = 0.11). However, BRV was faster when we excluded an outlier patient in part 1 (p = 0.0016). For our remaining primary outcome, parts 1 and 2 data combined, the median intrapatient BRV:LEV time ratio was 0.39 [95% confidence interval (CI) 0.16–0.91], i.e., PPR elimination was 61% faster with BRV, p = 0.039. PPR was completely eliminated in ≤ 2 min in 11 patients with BRV and in four patients with LEV. No period or carryover effects were seen. No serious or severe adverse effects occurred. At PPR elimination (n = 16), median plasma [BRV] was 250 ng/mL (range 30–4100) and median plasma [LEV] was 28.35 μg/mL (range 1–86.7).
Conclusion
Outcome studies directly comparing LEV and BRV are needed to define the clinical utility of the response with BRV, which was several minutes faster than that with LEV.
Clinical trials
ClinTrials.gov Identifier = NCT03580707; registered 07-09-18
Electronic supplementary material
The online version of this article (10.1007/s40263-020-00761-1) contains supplementary material, which is available to authorized users.
Key Points
| We compared the rapidity of central nervous system (CNS) effect for two related antiseizure medicines, brivaracetam (BRV) and levetiracetam (LEV), in patients with epilepsy with photosensitivity using the time to elimination of photoparoxysmal electroencephalogram response (PPR) post intravenous infusion as a pharmacodynamic endpoint. We employed a randomized, double-blind, two-period, balanced crossover design with equipotent doses of intravenous LEV 1500 mg and BRV 100 mg post-15-min and post-5-min intravenous infusions. |
| The median time to PPR elimination was 2 min for BRV versus 7.5 min for LEV for the 15-min and 5-min infusion rates and for both infusion rates combined. Statistical significance was not detected for the BRV:LEV time ratio to PPR elimination for two of the multiple primary outcomes (15-min and 5-min infusions analyzed separately). However, statistical significance was observed for another primary outcome, combined infusion data: the BRV:LEV time ratio was 0.39, indicating the CNS effect of BRV was 61% faster than that of LEV (p = 0.039). PPR was completely eliminated in ≤ 2 min in 11 patients with BRV versus four patients with LEV. |
| Our results confirm that BRV is pharmacologically distinguished from LEV. Studies are needed to further explore the clinical therapeutic significance of the apparent 5.5 min faster CNS penetrance with BRV. |
Introduction
The antiseizure medicines (ASMs) levetiracetam (LEV) and brivaracetam (BRV) are both approved by the US FDA for seizure treatment. BRV differs from LEV, both chemically and pharmacologically, with a ten-times greater affinity to the synaptic vesicle glycoprotein 2A (SV2A)-binding site than LEV (pKi = 7.1 and 6.1, respectively) [1, 2], ≥ 10- to 15-fold greater potency [3, 4], higher lipophilicity [4], and more potent protection than LEV against certain seizure types [5]. BRV may also act at different binding sites [5]. BRV penetrated the central nervous system (CNS) in audiogenic mice more quickly than did LEV [6], permitting faster BRV protection against clonic convulsions [6]. Positron emission tomography (PET) data in rhesus monkeys [6] and humans [7] showed greater and more rapid uptake (brain receptor occupancy) for BRV than LEV over time [6]. A post-hoc retrospective analysis [8] of separate LEV [9] and BRV [10] responses in studies of intermittent photic stimulation (IPS) in patients with photosensitive epilepsy suggested that BRV had a potentially earlier, greater pharmacodynamic photoparoxysmal response (PPR)-suppressive effect than LEV. These properties and cumulative data for BRV suggest that BRV may have clinical advantages over LEV, prompting our interest in conducting a direct head-to-head LEV versus BRV comparison study in patients to determine whether faster brain penetration could be achieved with BRV.
The human phase IIa photosensitivity model of epilepsy with IPS has been successfully used for years to identify the efficacy of single oral doses of potential new ASMs in epilepsy [11–15]. Typically, repeated hourly electroencephalogram (EEG) photosensitivity measures (baseline placebo day, then ASM day, within-patient, separate occasions) have been used. Both oral LEV [9] and BRV [10] suppressed the EEG PPR at 60 min in this model. However, for treatment of seizure emergencies or acute repetitive seizures, time to effect lies in the order of minutes after intravenous infusion of any ASM. Therefore, to assess differences in the time to effect of intravenous neuroactive ASMs, the model’s procedure needed to be repeated every few minutes, not over hours as per the conventional model. We adapted the model to compare these two intravenous ASMs in the same patient with time efficiency in three ways: (1) only studying the ASM-produced change in each volunteer patients’ EEG upper limit/threshold [16]; (2) limiting three eye conditions to the patient’s “best one,” based on each patient’s screening photosensitivity data; (3) eliminating some high Hz measurements, using each patient’s screening data.
With these adaptations to the conventional model, we devised a prospectively controlled, randomized, crossover, single-center study using serial measurements of the IPS-evoked EEG PPR as a pharmacodynamic efficacy endpoint over 2 h. Our hypothesis was that intravenous BRV would have faster peak EEG action and onset than intravenous LEV. Our primary objective was to determine the comparative (BRV to LEV ratio) time to PPR elimination or peak effect for equipotent doses of LEV (1500 mg) and BRV (100 mg) administered by intravenous infusion via multiple primary outcomes in patients with photosensitive epilepsy: (1) over 15 min (part 1), (2) then separately over 5 min (part 2), and (3) for combined time ratio data for parts 1 and 2. Our secondary objective was to determine any differential effect in onset of effect (time to diminution in each patient’s photosensitive response). An exploratory objective was to examine the number of patients who had a faster time to peak effect for BRV over LEV (if one existed) using descriptive metrics only.
Methods
Protocol
We conducted a prospective, computer-randomized, double-blind, two-period crossover study at a single site in patients with identified photosensitive epilepsy. Our protocol (see Fig. 1 in the electronic supplementary material [ESM]) had two parts. Part 1 involved a single intravenous infusion of LEV 1500 mg or an equipotent milligram dose of intravenous BRV 100 mg administered over 15 min (eight patients, crossed over to the other treatment arm no earlier than 1 week later). In part 2, the same single fixed milligram dose of LEV and BRV as in part 1 was given on separate occasions, randomly, via intravenous infusion over 5 min.1 The same patients participating in part 1 were allowed to enter part 2, but each patient had to wait a minimum of 2 weeks after their last drug infusion (i.e., > 14 days after part 1, visit 3 [second ASM infusion] before entering part 2, visit 2 [first ASM infusion]). Each part had two treatment periods, as per the balanced crossover design, with four patients randomized either to BRV first then to LEV or to LEV first then to BRV. For both parts, visits 2 and 3 were separated by a mandatory 14 days to allow for an adequate ASM-washout period. Table 1 in the ESM outlines the schedule/flowchart for all procedures/events required for patients in our study protocol.
Patient Screening and Accrual
All recruited patients were studied in a single center (Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, MO, USA). In total, 15 patients with genetic (idiopathic) generalized epilepsy plus PPR on previous EEGs were screened. Eight patient volunteers (five women; age 18–38 years; otherwise healthy per physical/neurological examination; normal laboratory results) were included. Their blinding codes were AAA, BBB, CCC, GGG, HHH, KKK, NNN, and OOO (in part 1). The ninth patient, PPP (female, age 42 years) replaced patient OOO in part 2. Thus, nine patients (six women, three men) participated/finalized all procedures (Table 2 in the ESM provides full clinical information). Patients completing part 1 had to meet all the same study inclusion criteria at re-screening to participate in part 2. Post-institutional review board (IRB) approval, we started recruitment for our study on 1 June 2018; the last patient visit in part 2 finished on 13 December 2018.
Intermittent Photic Stimulation (IPS) and Electroencephalogram (EEG) Data Acquisition
Our IPS procedure followed standardized recommendations [17] for eliciting/identifying PPRs via scalp EEG. We used a Grass PS-33-Plus Photic-Stimulator for IPS, performed every 5 s (less often when a generalized PPR occurred) during eye closure, with eyes closed, and with eyes open. The most effective baseline eye condition was selected for use during the two ASM administration trial days (see Table 2 in the ESM for detailed IPS procedures and pertinent baseline EEG/IPS conditions). Patient EEG data obtained (by WER, who was blinded) was reviewed by the blinded central reader for EEG (DKNT) post-data collection. If differences in EEG interpretations between WER and DKNT occurred, consensus was reached by re-reading the EEG and video files at the site while maintaining the blind.
Levetiracetam (LEV)/Brivaracetam (BRV) Intravenous Injection Preparation and Administration: Blood Sampling for [LEV] and [BRV] Analyses
Intravenous LEV via infusion is only FDA approved for infusion at a rate no faster than 15 min (per manufacturer’s package insert [42]). We evaluated the rapidity of EEG effect from equipotent, fixed doses of LEV 1500 mg compared with BRV 100 mg given intravenously over 15 min (study part 1), and on a separate occasion, as a 5-min infusion (part 2). We are aware that some clinicians are prepared to administer LEV as a 5-min intravenous infusion based upon perceived patient need for acute treatment of repetitive seizures plus published literature detailing clinicians’ favorable experiences (not being associated with hypotension or arrhythmias) with this administration [18], even in critically ill patients [19–22]. (Since the completion of our protocol, three other sentinel works have been published in patients with status epilepticus [SE] using LEV infusion safely over 5 min [23, 24] or 10 min [25]). BRV was also infused over 5 min to match the LEV time of administration and to allow for study blinding with direct comparison of results. We filed an Investigational New Drug (IND) application notifying the FDA of our intent to use a 5-min intravenous LEV infusion. Although BRV infusion is FDA approved for intravenous bolus over as little as 2 min, published information for LEV administered at this rate is lacking, so we did not study this option.
To simplify the intravenous preparation on test administration visit days, we intentionally chose to administer equipotent fixed milligram LEV and BRV doses rather than weight-based milligram dosing to patients. We surmised that we could compare LEV and BRV plasma concentrations at various times concurrent with EEG effects, as if mg/kg doses were given, via dose normalization to 70 kg in post-hoc analysis, since the pharmacokinetics for both LEV [18] and BRV [26] are linear.
All staff were blinded to LEV/BRV randomization, except for RCR and the unblinded physician (SML) who prepared the assigned ASM injections (SML maintained the blinding sequence using sealed envelopes marked “SML access only” and kept in a locked safe). Commercial LEV/BRV was admixed with 0.9% normal saline per package insert. In part 1, the intravenous LEV or BRV dosage was delivered by zero-order infusion pump (Baxter-6201) over 15 min (rate 460 mL/h), enabling 115 mL fluid over 15 min. Then, 0.9% intravenous sodium chloride or normal saline 10 mL was injected into the tubing to flush out any ASM left in the tubing line. In part 2, 115 mL ASM-containing fluid was given over 5 min (rate 1380 mL/h), followed by saline. One patient (AAA) in part 2, option 2, received a lower dosage of LEV and BRV, on separate occasions over 15 min (see the ‘Expanded Descriptions of Select Items from Manuscript Text’ in the ESM for more detailed information).
Prior to starting IPS, 18-gauge indwelling catheters were placed into an antecubital vein (one in each arm). One catheter was used to administer intravenous LEV or BRV; the contralateral arm was used for blood sample acquisition. Multiple blood samples for LEV and BRV determination were obtained concurrent with IPS testing.
Two distinct proprietary liquid chromatography-mass spectrometry/mass spectrometry methods were used for determining plasma [LEV] and [BRV] concentrations, respectively. The lower limit of detection (LloD) for LEV was 1.0 μg/mL, with a % coefficient of variation (%CV) of 10% at LloD; the LloD for BRV was 0.1 μg/mL (100 ng/mL), with a 3.1% within-day %CV.
Patient Safety Endpoints
A complete medical history, physical examination, and blood laboratory tests were carried out at the start of the study and repeated at the final visit day (Table 1 in the ESM). During screening and the complete trial, patients were continuously monitored by medical personnel; vital signs and physical and neurological examinations were performed during confinement periods. All adverse events (AEs), treatment-emergent AEs, and serious AEs were noted. The IPS was performed in a standardized manner [17] to prevent unnecessary seizure provocation [27].
Sample Size Calculation and Statistical Analysis
Our a priori sample size calculation was based upon deriving an answer for our first study objective, log (time) to PPR elimination (i.e., peak EEG effect), based on a two-period balanced crossover study. With a sample size in each sequence group of four (total sample of eight), we calculated that a 2 × 2 crossover design would have 80% power to detect a difference in log time (to PPR elimination) means of − 1.20, assuming that the crossover analysis of variance (ANOVA) root mean square error (MSE) is 0.707 (the standard deviation of differences is 1.00) using a two group t-test (crossover ANOVA) with a 0.05 two-sided significance level. A difference of − 1.200 in log time (to PPR elimination) corresponds to an intrapatient ratio of time of exp (− 1.2) = 0.3, i.e., a 70% reduction in time. Patients not completing the crossover for part 1 or part 2 were to be replaced. We planned a priori to have multiple (three) outcomes for our first objective: to analyze our data separately per part as well as for the two parts combined, using a linear mixed model on the log time (to PPR elimination). We assumed that if no statistical significance was found for parts 1 and 2 separately, combining data from both parts would be justified since most physicians do not distinguish between 5- and 15-min ASM infusions when treating patients clinically for acute seizure clusters or acute repetitive seizures. The mixed models for parts 1 and 2 contained a term to detect a period effect, the sequence variable as a term to detect a carryover effect (the sequence variable, BRV first and LEV second, or vice versa, i.e., after crossover to the other treatment), and the treatment indicator (BRV or LEV) as fixed effects and patient ID as random intercept effect. After excluding evidence of a period effect and a carryover effect, the model was simplified to have only the treatment indicator as fixed effect. The mixed model for the simultaneous analysis of parts 1 and 2 combined contained the same terms plus a term to identify the study part (1 or 2), to account for a systemic difference between the two parts, and an interaction term between treatment and study part, to test whether the treatment effect was the same in each. The random part of the combined analysis contained a random intercept and random effect of part, allowing for a difference in variation in response between the parts. The statistical significance of the difference in log (time) between BRV and LEV was determined from the linear mixed models using the t-test with the Satterthwaite approximation, as implemented in the LmerTest package in R, as this correction method has been shown to be not “anticonservative” in small sample sizes [28].
The second objective, time to onset of PPR diminution by each ASM, was to be analyzed by a paired t-test. We planned a descriptive analysis only for the number and percentage of patients with a difference in time to peak response between LEV and BRV, since any statistical analysis on number or percentage of patients would merely default to the same statistical test executed for examining our first objective.
All pertinent EEG data were logged into a secured database (Microsoft Access), and R version 3.5.0 (R Core Team [2018]; https://www.R-project.org/) was used for statistical analysis.
Results
Patient Recruitment, Physical and Clinical Characteristics, Concomitant Antiseizure Medicines
Patient recruitment started on 1 June 2018 and was completed by 19 November 2018. Table 2 in the ESM lists the pertinent clinical and EEG PPR characteristics for randomized patients, including seizure type, age, and stable comorbidities (if present). All study patients met the minimum required 14-day washout time between visits. For part 1 then 2, respectively, the average number of days between visit 2 and 3 ASM administration was 17.4 (range 14–21) and 15.9 (range 15–20). The average number of days from visit 3 (part 1) to the next ASM administration (visit 2, part 2) was 112.1 (range 29–167).
Concomitant ASM plasma concentrations [ASM] were obtained for ASMs routinely measured at screening and on LEV or BRV intravenous administration days, visits 2 and 3 (Table 3 in the ESM). We retrospectively analyzed plasma concomitant [ASM] concentration results (measured in local clinical laboratories) to identify large or unexpected variations in plasma [ASM], i.e., medication adherence (a zero plasma [ASM] would indicate nonadherence). No patient was deemed nonadherent; [ASM] concentrations were relatively stable over time.
One patient (AAA) had a generalized tonic–clonic (GTC) seizure on the morning of visit 2, part 1, before study drug administration. The responsible medical investigators (WER, SML) determined that the patient had recovered and could proceed with the study later that day. The GTC seizure was not deemed to be an AE, as the patient had a history of GTC seizures.
General EEG Results
The following observations were made for the 32 randomized patient visits with either BRV or LEV infusions.
All patients were responsive to both intravenous BRV and LEV, except that patient NNN did not display PPR elimination or a robust BRV response within 120 min in part 1 but responded swiftly to BRV in part 2 at 1 min).
Time to peak EEG effect or PPR elimination was prompt in all patients (except 1 in 32 EEG scorings, patient NNN). When PPR elimination occurred, it did so completely for all flash frequencies tested.
One patient (AAA) was placed into option 2 (lower ASM doses) in part 2 after showing no difference in time to PPR elimination for LEV versus BRV in part 1 (see the ESM for details).
It was not possible to statistically attain the onset of effect, i.e., time to PPR diminution, because PPR elimination occurred so promptly in both parts 1 and 2 for both BRV and LEV.
No statistically significant period sequence or carryover effects were observed.
Fig. 2 in the ESM depicts an example of a patient’s EEG with a PPR present at baseline (panel ‘A’), then PPR elimination with BRV at 1 min into infusion (panel ‘B’). Sustained PPR elimination continuing at 20 min into the 15-min infusion is not shown.
Time to Photoparoxysmal Response (PPR) Elimination on EEG (Primary Study Objective)
Table 1 lists the time to PPR elimination, which was remarkably quick for BRV in part 1 (median 2 min into the start of the 15-min linear BRV intravenous infusion) and quick for LEV in part 1 (median 7.5 min). Likewise, the time to PPR elimination was remarkably quick for BRV in part 2 (median 1.5 min into the 5-min linear BRV intravenous infusion) and quick for LEV in part 2 (median 7.5 min into the 5-min linear LEV intravenous infusion). Overall, we observed that BRV eliminated the PPR more quickly than did LEV (median 2.0 min for BRV and 7.5 min for LEV, representing an absolute difference in median time to PPR elimination of 5.5 min in favor of BRV) (Table 1).
Table 1.
Numerical listing of time to peak EEG effect (i.e., PPR elimination, min) for intravenous levetiracetam and brivaracetam in part 1 (over 15 min), part 2 (over 5 min), and parts 1 and 2 combined
| Time (min) to PPR elimination | AAA | BBB | CCC | GGG | HHH | KKK | NNN | OOO,a, PPP | Median (range) |
|---|---|---|---|---|---|---|---|---|---|
| LEV | |||||||||
| Part 1 | 2 | 5 | 30 | 15 | 10 | 10 | 5 | 5 | 7.5 (2–30) |
| Part 2 | 15 | 60 | 2 | 1 | 1 | 5 | 30 | 10 | 7.5 (1–60) |
| Parts 1 and 2 | – | – | – | – | – | – | – | – | 7.5 (1–60) |
| BRV | |||||||||
| Part 1 | 2 | 2 | 5 | 1 | 1 | 2 | ≥ 120 | 1 | 2 (1–120) |
| Part 2 | 1 | 1 | 5 | 10 | 1 | 5 | 1 | 2 | 1.5 (1–10) |
| Parts 1 and 2 | – | – | – | – | – | – | – | – | 2 (1–120); (1–5b) |
Part 1, n = 8; part 2, n = 8; parts 1 and 2, n = 16
BRV brivaracetam, EEG electroencephalogram, LEV levetiracetam, PPR photoparoxysmal response
aPatient OOO completed part 1, but patient PPP participated in part 2
bRange when excluding patient NNN
The data in Table 1 are informative and clear, but proper comparison of the intrapatient response data in our crossover study required the use of the BRV:LEV ratio value for time (min) to PPR elimination, with statistical comparison via the t-test with the Satterthwaite approximation on the linear mixed model. Here, a BRV:LEV ratio value of 1.0 indicated the equivalence of the two ASMs, a value < 1.0 indicated a faster response for BRV, and a value > 1.0 indicated that LEV was faster. Table 2 lists each BRV:LEV time-to-PPR-elimination ratio value for each patient. The results for two of our primary outcomes, part 1 and part 2 examined separately, were not statistically significant. When examining part 1 alone, the median intrapatient BRV:LEV time-to-PPR-elimination ratio value was 0.44 [95% confidence interval (CI) 0.13–1.49]; p = 0.22. However, without nonresponder patient NNN (for BRV in part 1), the median intrapatient BRV:LEV ratio value was 0.25 (95% CI 0.14–0.44); p = 0.0016. For part 2 alone, the BRV:LEV ratio value was 0.34 (95% CI 0.099–1.18); p = 0.11. Yet, a statistically significant intrapatient BRV:LEV ratio value was observed for our prespecified third outcome, parts 1 and 2 data combined, at 0.39 (95% CI 0.16–0.91); p = 0.039 (note: n = 16 calculated time ratios, representing seven patients participating in both parts [including patient NNN in part 1] plus one patient each in part 1 [OOO] and part 2 [PPP]). The test for the interaction of treatment with part was not significant (p = 0.79). Figure 1 displays individual and median intrapatient BRV:LEV ratio values for time to PPR elimination. Pharmacodynamic variability was greater in the BRV:LEV ratio of time to EEG peak suppression or PPR elimination for patients in part 2 compared with part 1, as visualized in Fig. 1.
Table 2.
EEG summary results for levetiracetam vs. brivaracetam, parts 1 and 2, and parts 1 and 2 combined, reported as intrapatient BRV:LEV ratio value for time to peak EEG effect (PPR elimination, in min)a
| Treatment randomization sequence | Treatment period | Intrapatient (BRV:LEV) ratio for time to PPR elimination (min) | |
|---|---|---|---|
| 1 (or visit 2) | 2 (or visit 3) | ||
| Part 1: 15-min IV | Time to PPR elimination (min) | ||
| BRV then LEV | |||
| AAA | 2 | 2 | 1 |
| BBB | 2 | 5 | 0.4 |
| KKK | 2 | 10 | 0.2 |
| OOO | 1 | 5 | 0.2 |
| LEV then BRV | |||
| CCC | 30 | 5 | 0.167 |
| GGG | 5 | 1 | 0.2 |
| HHH | 10 | 1 | 0.1 |
| NNN | 5 | 120 | 24 |
| Part 1 median BRV:LEV ratio (n = 8) | 0.44 (95% CI 0.13–1.49); p = 0.22 | ||
|
Part 1 median without nonresponder patient NNN (for BRV) (n = 7) |
0.25 (95% CI 0.14–0.44); p = 0.0016 | ||
| Part 2: 5-min IV | |||
| BRV then LEV | |||
| AAA | 1 | 15 | 0.0667 |
| HHH | 1 | 1 | 1 |
| KKK | 5 | 5 | 1 |
| NNN | 1 | 30 | 0.0333 |
| LEV then BRV | |||
| BBB | 60 | 1 | 0.0167 |
| CCC | 2 | 5 | 2.5 |
| GGG | 1 | 10 | 10 |
| PPP | 10 | 2 | 0.2 |
| Part 2 median BRV:LEV ratio (n = 8) | 0.34 (95% CI 0.099–1.18); p = 0.11 | ||
| Parts 1 and 2 BRV:LEV ratio combined, median (n = 16) calculated time ratios (includes outlier patient NNN) | 0.39 (95% CI 0.16–0.91); p = 0.039 | ||
BRV brivaracetam, CI confidence interval, EEG electroencephalogram, IV intravenous infusion, LEV levetiracetam, PPR photoparoxysmal response
aA BRV:LEV intrapatient ratio value = 1.0 means no difference between the two ASMs; < 1.0 indicates a faster time to PPR elimination for BRV, and a ratio value > 1.0 denotes a faster LEV response
Fig. 1.
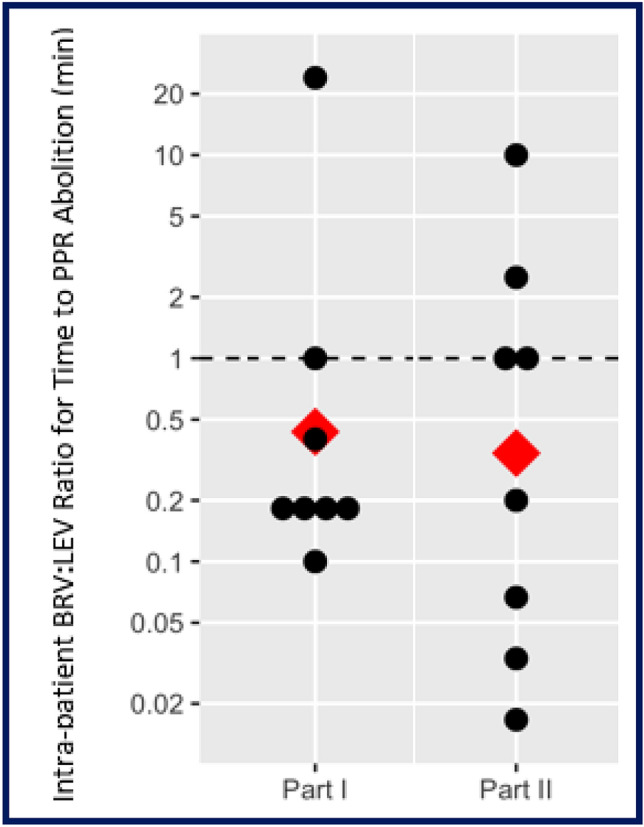
The separate dot plots for part 1 and part 2 are shown for the intrapatient BRV:LEV ratio values for time to PPR elimination on EEG in patients with photosensitive epilepsy. Individual BRV:LEV intrapatient time ratios are denoted by the black dots, and the median for the group of patients per part by the red diamond. A BRV:LEV time ratio of unity (value = 1.0, dotted horizontal line) means no difference in time to PPR elimination between the two ASMs; a ratio value < 1.0 indicates a faster time to PPR elimination for BRV over LEV, a value > 1.0 denotes that LEV was faster than BRV. For part 1 (n = 8), the median BRV:LEV time ratio value was 0.44, p = 0.22. Patient NNN was the outlier in part 1 with a BRV:LEV time ratio > 20; excluding patient NNN (n = 7) yielded a median BRV:LEV time ratio value of 0.25, p = 0.0016. For part 2 (n = 8), the time ratio value was 0.34, p = 0.11, and greater pharmacodynamic variability was observed. When parts 1 and 2 were combined (data not shown, n = 16), the median BRV:LEV time ratio value was 0.39, p = 0.039, indicating a statistically significantly faster time to peak effect for BRV over LEV. ASM antiseizure medicine, BRV brivaracetam, EEG electroencephalogram, LEV levetiracetam, PPR photoparoxysmal response
Onset of Effect for PPR Diminution and Number of Patients Exhibiting Faster BRV Response
Our second study objective, time to onset of EEG effect, could not be accurately derived here because of the rapidity and completeness of ASM peak effect. Both LEV and BRV displayed quick electrophysiological action after 15- or 5-min intravenous administration. It was so quick, in fact, that reliably capturing the actual onset of effect on EEG was not possible. All PPR suppression occurred before or at 30 min in 30 of 32 instances (patient BBB at 60 min for LEV in part 2, and patient NNN had no response) or at ≥ 120 min for BRV in part 1 (Table 1).
We undertook an exploratory analysis to examine the number and percentage of patients exhibiting a faster time to peak EEG effect/PPR elimination between the two ASMs. When separating the number of patients who displayed PPR elimination at clinically popular milestones of ≤ 2, or within 5, or at ≥ 10 min, we found that BRV eliminated the PPR more quickly than LEV on more occasions (Table 3). For all results, a response was seen with BRV within 2 min in 11 of 16 episodes (69%) compared with 4 of 16 patients (25%) for LEV; the BRV then LEV patient episode numbers were similar at the 5-min milestone. We noted that LEV produced a response at the later time of ≥ 10 min in 8 of 16 episodes (50%), whereas only 2 of 16 (12%) BRV-treated patient episodes had PPR elimination at this later time.
Table 3.
Number and percentage of patient episodes with PPR elimination on EEG for BRV and LEV at ≤ 2, 5, and ≥ 10 min time points in patients with photosensitive epilepsy
| ASM (n observations, parts 1 and 2) | Number of occasions (% of patients) with PPR elimination at designated time points: | |||
|---|---|---|---|---|
| ≤ 2 min | 5 min | ≥ 10 min | ||
| BRV (n = 16) | 11 (69%) | 3 (19%) | 2 (12%) | |
| LEV (n = 16) | 4 (25%) | 4 (25%) | 8 (50%) | |
| ASM (n observations, part 1 only) | BRV (n = 8) | 6 (75%) | 1 (12.5%) | 1 (12.5%) |
| LEV (n = 8) | 1 (12.5%) | 3 (37.5%) | 4 (50%) | |
| ASM (n observations, part 2 only) | BRV (n = 8) | 5 (62.5%) | 2 (25%) | 1 (12.5%) |
| LEV (n = 8) | 3 (37.5%) | 1 (12.5%) | 4 (50%) | |
ASM antiseizure medicine, BRV brivaracetam, CI confidence interval, EEG electroencephalogram, IV intravenous, LEV levetiracetam, PPR photoparoxysmal response
Plasma [LEV] and [BRV] Concentration Data at the Time of PPR Elimination
The median plasma [LEV] concentration at the time point for PPR elimination for parts 1 and 2 combined was 28.35 μg/mL (range 1–86.7). The median plasma [BRV] concentration at PPR elimination for combined data was 250 ng/mL (range 30–4100).
Patient Safety
Overall, AEs included mild transient lightheadedness that occurred in four BRV-treated patients and two LEV-treated patients; drowsiness occurred in four LEV patients. No severe or serious FDA-reportable AEs occurred. No real difference was discerned between part 2 (5-min intravenous infusion) and part 1 (15-min intravenous infusion) in the number, type, or severity of AEs (the rate of AEs was so low). A 5-min intravenous infusion did not appear to produce an increase in AE burden in our nine patients.
Discussion
Both LEV and BRV have previously been shown to eliminate the PPR in patients with epilepsy in their respective early drug-development proof-of-concept phase IIa studies [9, 10]. Despite FDA approval, LEV and BRV have not yet undergone formal head-to-head studies in patients with epilepsy.
Our primary objective was to investigate whether BRV displayed a faster time to PPR elimination on EEG (i.e., peak EEG effect) than LEV using a randomized, double-blinded, crossover design. When combining all data, BRV eliminated PPR in a median of 2 min; LEV took 7.5 min (Table 1). In this crossover study, the optimal statistical analysis for time to peak effect required use of the mixed-model analysis for the intrapatient BRV:LEV time ratio for part 1, then part 2, and for combined parts 1 and 2. As seen in Table 2, it was only when patient NNN, an extreme outlier with no response to BRV in part 1, was excluded that we found statistical significance in favor of BRV (median intrapatient BRV:LEV ratio 0.25 [95% CI 0.14–0.44], p = 0.0016). Part 2 alone showed greater variability in response for both LEV and BRV (Table 2, Fig. 1). Again, a test for the interaction of treatment with part was not significant (p = 0.79); accordingly, parts 1 and 2 were combined (16 calculated time ratios in nine patients, including outlier patient NNN). BRV was statistically faster to eliminate PPR, as evidenced by an intrapatient BRV:LEV time ratio of 0.39 (95% CI 0.16–0.91), p = 0.039 (Table 2). In the absence of clinical data to evaluate the efficacy and/or safety of 5- and 15-min ASM infusions, our impression is that most clinicians would not differentiate a 10-min difference in ASM infusion times, focusing on the idea that “faster is better” in their routine clinical practice for optimal management of the acutely seizing patient, further justifying the use of combined data for the 15- and 5-min infusion rates.
Clinician confidence in the efficacy of LEV is evident by its use beyond that of outpatient management of seizures. An evidence-based review of efficacy data supported the preferential use of LEV in patients with either focal or GTC seizures [29] or for SE [30] or even for refractory convulsive SE [31]. In a large Cochrane database review [32], LEV was equally as effective as lorazepam in aborting seizures from SE. Likewise, other clinical experience and evidence with intravenous LEV 1000 mg in patients with SE resistant to benzodiazepines shows an efficacy rate of 70% [33, 34]. Moreover, two recently reported large, open-label, multicenter, randomized trials suggested that intravenous LEV is an appropriate alternative to phenytoin for convulsive pediatric SE [23, 24], and a third fully blinded randomized trial of intravenous LEV compared with fosphenytoin and valproate in benzodiazepine-refractory SE found similar efficacy rates (~ 50%) and AEs amongst these three ASMs [25]. Considering the popularity of LEV, some clinicians have begun conducting open-label studies of BRV (not compared with LEV) for SE [35–39]. This overall efficacy rate of 50% [25] to 70% [30] for the treatment of SE by ASMs is still not considered optimal, prompting calls for better ASM treatments for SE. One of the key issues in the treatment of SE is time to effect to reduce the negative impact on brain function of prolonged ictal discharges. Oral adjunctive BRV has shown good efficacy in clinical trials for focal epilepsies [40, 41] and therefore has received FDA approval. Thus, it is to be expected that BRV might especially diminish the time to effect in patients with acute seizures or SE.
Our deliberate choice of total milligram intravenous single doses for LEV 1500 mg versus BRV 100 mg deserves special mention. Several authors have claimed that BRV has between a 10- and 15-times greater potency than LEV [3, 4]; notably, we chose to use the data claiming 15 times greater potency for BRV, since it fits our conservative approach. If we had assumed a lesser (10 ×) potency for BRV, it would have required that we use more intravenous BRV (i.e., 150 mg) versus intravenous LEV 1500 mg in our study. Furthermore, when considering PET data from human brain [7], our choice of BRV 100 mg was certainly conservative and less biased in favor of BRV, since the PET tracer displacement half-times for BRV are dose dependent: SV2A PET tracer displacement half-times were longer and SV2A receptor occupancy lower for lower milligram doses of BRV. The drug entry half-time into brain and percent of SV2A receptor occupancy from an intravenous BRV dose of 100 mg averages 10 min and 68%, whereas it is 1.9 min and 84.5% for an intravenous BRV dose of 200 mg, respectively [7]. Hence, we intentionally chose to use a BRV dose based on the 15-times greater potency over LEV, reducing the total milligram BRV dose equivalent administered, since it was a more conservative estimate for BRV and perhaps less “biased” in favor of BRV.
Likewise, our deliberate choice of comparative intravenous infusion rates also deserves special mention. LEV has only been FDA approved for an intravenous infusion rate no faster than 15 min [42], yet, many clinicians have chosen to give intravenous LEV more quickly as a 5-min intravenous infusion, with reported safety and tolerability [18], even in the critically ill patient [19–24]. For purposes of this protocol, we filed an IND application form 1571 with the FDA, informing them of our intent to administer intravenous LEV “off label” over a 5-min infusion period. However, injectable BRV is FDA approved for an intravenous infusion rate of 2–15 min [43]. As such, we devised our protocol to compare a single intravenous equipotent milligram dose of LEV and BRV head to head in crossover fashion on separate occasions, first (part 1) over a 15-min intravenous period and then (part 2) over a 5-min intravenous infusion period. Published safety data for intravenous LEV infusion over a 2-min period was too limited at the time of the start of our protocol, so we chose not to directly compare these to ASMs at the 2-min intravenous infusion rate. The use of BRV at a higher dose than used here (> 100 mg) and at a 2-min intravenous infusion rate compared with the conventional LEV dose and intravenous infusion rate deserves further study.
In summary, our results for combined infusion are in line with much faster CNS tissue penetration (ng/g brain) with BRV than with LEV [6]. Together with findings from PET studies in both rhesus monkeys [6] and human volunteers [7], which showed SV2A occupancy was faster and greater with BRV than with near equipotent LEV after intravenous injection, our results support the use of BRV in the treatment of acute seizure conditions. Our finding of a faster time to peak EEG effect with intravenous BRV than with LEV, meaning a 61% faster entry time for BRV (p = 0.039, Table 2 and Fig. 1), needs to be confirmed and explored further clinically, since we do not know the clinical significance of the median difference of 5.5 min (Table 1). We note the majority of patients had a more rapid EEG response with BRV than with LEV (Table 3). Several recent large, adequately powered randomized trials evaluated intravenous LEV compared with phenytoin [23, 24] or conducted a three-way evaluation of LEV compared with valproate or phenytoin in patients with SE [25] and found no evidence for superiority or inferiority among the treatments. Considering the experimental data that clearly distinguish BRV from LEV [1–7], and our data herein showing faster EEG pharmacodynamic effect in patients with photosensitive epilepsy, perhaps the next step is for testing of BRV against other intravenous ASMs in a formal randomized clinical outcomes trial.
Study Limitations
This study has some limitations that must be considered. First, our sample size was small at eight patients per part; however, we designed the study as an intrapatient crossover study to increase its power. We had planned for a 70% reduction in BRV:LEV time ratio but observed a lesser 61% faster BRV response. We also encountered more response variability than projected. Accordingly, our statistical significance for greater rapidity of effect with BRV versus LEV was only seen after combining patient data from both infusion rates. Second, we conducted IPS testing only six times in the first 20 min after the start of the intravenous infusion. We note that the peak PPR effect after oral dosing for LEV [9] and BRV [10] essentially occurred concurrently with peak plasma [ASM] concentration at 60 min, and we had surmised that our IPS testing frequency would have been sufficient to capture essential data. In retrospect, we could have IPS tested more frequently, perhaps every 1–1.5 min in that time period to capture differences in time to peak EEG effect for the two ASMs even more precisely. Third, we did not measure BRV or LEV concentrations in cerebrospinal fluid over time, which might have helped us understand the differences in time to LEV versus BRV pharmacodynamic effect. Fourth, no head-to-head comparative clinical study for LEV versus BRV exists to provide guidance for equipotent dosing. Such a study, if it had existed, could have provided information regarding an optimal dose to use for comparison of intravenous LEV versus BRV. Instead, we used an intravenous LEV dose of 1500 mg based on a previous LEV photosensitivity study [9] where 1000 mg was sufficient, but not optimal, to eliminate the PPR, and then we relied on suitable in vitro and animal experimentation data [3, 4, 7] for our choice of a reasonable equipotent intravenous BRV dose of 100 mg. Fifth, it is possible that a very large intravenous LEV dose, e.g., 4500 mg, such as administered in the ESETT study [25], could very rapidly flood brain SV2A receptors, thus lessening the time to PPR elimination compared with lower doses. A higher intravenous LEV dose deserves to be compared with an equipotent intravenous BRV dose in a photosensitivity study of similar design to ours. Finally, we limited our study to two intravenous infusion rates for a fair comparison. Dosing intravenous BRV at the approved 2-min infusion time was beyond the scope of this protocol, and this deserves further study in comparison with conventional intravenous LEV dosing.
Conclusions
BRV eliminated the pharmacodynamic EEG biomarker of photosensitivity in patients with epilepsy, the PPR, more quickly than did LEV; the median time to PPR elimination with BRV was 5.5 min faster than with LEV post-intravenous infusion at presumed equipotent milligram doses. No statistically significant difference for BRV:LEV time ratio to PPR elimination was seen for 15- and 5-min infusion rates when analyzed separately, but statistical significance was observed for combined data. Clinical outcome studies directly comparing intravenous LEV with BRV are needed to confirm our findings and to further clinically differentiate LEV and BRV.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
All authors are indebted to Armel Stockis, PhD, UCB Clinical Development; Melinda Martin, PhD; Prashant Dongre, MD; and the late Joseph D’Souza, all UCB, for their considerable interest and valuable procedural investigator-initiated study guidance.
Declarations
Funding
UCB Pharma did fund our IRB-approved Investigator-Initiated Study (IIS), but did not participate in the design of the protocol, execution of the IIS, data acquisition/analysis, nor generation/revision of our manuscript. The WVU Arthur I. Jacknowitz Foundation Endowment Fund provided partial support for investigator/author RCR and paid the ‘open access fee’ for our manuscript in CNS Drugs in full.
Conflicts of Interest
RCR and his research efforts were supported, in part, by the WVU Arthur I. Jacknowitz Endowment Fund. RCR has acted as a consultant for Otsuka Pharmaceuticals and UCB. WER has acted as a consultant/advisor for SK Life Science, Inc.; acted as a speaker for Eisai, Greenwich Biosciences (formerly GW Pharmaceuticals), SK Life Science, Inc., Sunovion, and UCB; and has received research support from Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Inc., Takeda, UCB, and Upsher-Smith. SML has received research support from Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Inc., Takeda, UCB, and Upsher-Smith. RMJCE has received research support from UCB for statistical analysis for this project only. DGAKNT has received research support from UCB and Otsuka Pharmaceuticals and lecture honoraria from UCB, Otsuka Pharmaceuticals, and SK Pharma.
Ethics Approval
Our protocol was reviewed and approved by Schulman and Associates, Protocol SAIRB-18–0016, version 1 –8 January 2018, Amendment 1 – 6 April 2018, under the title: “Does brivaracetam (BRV) have a faster onset time and greater effect than levetiracetam (LEV) in epilepsy patients?: A prospective, randomized, crossover, double-blind, controlled intravenous study using the evoked photoparoxysmal EEG response (PPR) as a pharmacodynamic efficacy endpoint.” Our letter from Schulman and Associates dated 2 May 2018, indicated our protocol (reference IRB #201802030) had full board approval on 27 April 2018, with expiration on 26 April 2019.
Consent to Participate
Separate consent forms for parts 1 and 2 of our protocol were approved by Schulman and Associates. All patients provided written informed consent prior to any research activities being conducted (at the beginning of the screening visit).
Consent for Publication
Not applicable.
Availability of Data and Material
Readers are reminded that some material is available in the online Electronic Supplementary Material (ESM). All original patient records and original EEG tracings/video files are kept by WER and SML. Final EEG analysis of original EEG data is kept by DKNT. Original statistical analysis data is kept by MJCE-(Rene). Original plasma drug concentration data, recalculated/reformatted drug concentration data, and data sets that generated all our Tables and Figure(s) for our current study/manuscript are kept by the Corresponding Author (RCR). Reasonable requests for data from the authors can be made at their addresses listed on the last page (Affiliations section), or via email from the corresponding author, RCR.
Availability of Code
All pertinent EEG data was logged into a secured database (Access). R version 3.5.0 (R Core Team [2018]; https://www.R-project.org/) was used for statistical analysis. The Satterthwaite approximation (correction method for comparison of groups of small sample size in the linear mixed model) can be found the LmerTest package in R.
Author Contributions
RCR and DGAKNT conceptualized and wrote the original study design; all authors contributed to study modifications, and the final protocol was approved by all. Two simulations/mock runs of all study procedures were conducted by all authors (except for RMJCE) and research staff at the clinical study site (Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, MO, USA) before the screening of the first patient. The statistical plan was written by RMJCE, who also carried out the statistical interpretation and analysis. RCR and DKNT visited the clinical site on several occasions, during both parts 1 and 2, to advise on data collection and conduct quality assurance. Blinded EEG analysis, post-patient visits, was performed by DKNT. RCR performed the pharmacokinetic analysis. Material was prepared, data were collected, and patient clinic visits were conducted by WER and SML. Data were analyzed by RCR, DKNT, WER, and RMJCE. The first draft of our manuscript was written by RCR; comments and critical revisions for important intellectual content in all subsequent version(s) were made by all authors. All authors read the reviewers’ and editor’s comments, submitted their proposed alterations, and approved this final revised manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Part 2 had two options. Readers are referred to the ESM, “Expanded descriptions of select items from manuscript text” for more details on the only patient, AAA, to qualify for a different option.
References
- 1.Kenda BM, Matagne AC, Talaga PE, Pasau PM, Differding E, Lallemand BI, et al. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47:530–549. doi: 10.1021/jm030913e. [DOI] [PubMed] [Google Scholar]
- 2.Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anticonvulsant properties. Eur J Pharmacol. 2011;664:36–44. doi: 10.1016/j.ejphar.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 3.Matagne A, Margineanu D-G, Kenda B, Michel P, Klitgaard H. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154:1662–1671. doi: 10.1038/bjp.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klitgaard H, Matagne A, Nicolas J-M, Gillard M, Lamberty Y, De Ryck M, et al. Brivaracetam: rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia. 2016;57(4):538–548. doi: 10.1111/epi.13340. [DOI] [PubMed] [Google Scholar]
- 5.Wood MD, Gillard M. Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. 2017;58(2):255–262. doi: 10.1111/epi.13638. [DOI] [PubMed] [Google Scholar]
- 6.Nicolas JM, Hannestad J, Holden D, Kervyn S, Nabulsi N, Tytgat D, et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia. 2016;57(2):201–209. doi: 10.1111/epi.13267. [DOI] [PubMed] [Google Scholar]
- 7.Finnema SJ, Rossano S, Naganawa M, Henry S, Gao H, Pracitto R, et al. A single-center, open-label positron emission tomography study to evaluate brivaracetam and levetiracetam synaptic vesicle glycoprotein 2A binding in healthy volunteers. Epilepsia. 2019;60(5):958–967. doi: 10.1111/epi.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed RC, Kasteleijn-Nolst Trenité DGA. Do levetiracetam (LEV) and brivaracetam (BRV) differ in their effect on the photoparoxysmal EEG response (PPR) in epilepsy patients?: Results of a retrospective, data mining study. Epilepsia. 2014;55(Suppl 2):181–182. [Google Scholar]
- 9.Kasteleijn-Nolst Trenité DG, Marescaux C, Stodieck S, Edelbroek PM, Oosting J. Photosensitive Epilepsy: A model to study the effects of antiepileptic drugs. Evaluation of the piracetam analogue, Levetiracetam. Epilepsy Res. 1996;25(3):225–230. doi: 10.1016/S0920-1211(96)00031-9. [DOI] [PubMed] [Google Scholar]
- 10.Kasteleijn-Nolst Trenité DG, Genton P, Parain D, Masnou P, Steinhoff BJ, Jacobs T, et al. Evaluation of Brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology. 2007;69(10):1027–1034. doi: 10.1212/01.wnl.0000271385.85302.55. [DOI] [PubMed] [Google Scholar]
- 11.Binnie CD, Kasteleijn-Nolst Trenité DGA, De Korte RA. Photosensitivity as a model for acute antiepileptic drug studies. Electroencephalogr Clin Neurophysiol. 1986;63:35–41. doi: 10.1016/0013-4694(86)90060-x. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt B. Proof of principle studies. Epilepsy Res. 2006;68(1):48–52. doi: 10.1016/j.eplepsyres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Yuen ES, Sims JR. How predictive are photosensitive epilepsy models as proof of principle trials for epilepsy? Seizure. 2014;23(6):490–493. doi: 10.1016/j.seizure.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Kasteleijn Nolst-Trenité DG, Genton P, Brandt C, Reed RC. The ‘Photosensitivity Model’ is (also) a model for partial focal (partial) seizures. Epilepsy Res. 2017;133:113–120. doi: 10.1016/j.eplepsyres.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Kasteleijn-Nolst Trenité DGA and Reed RC. False Positive or False Negative Results do not exist in the Human Photosensitivity Phase IIa Model. 72nd Annual Meeting of the American Epilepsy Society (AES), November 30th–December 4th, 2018, New Orleans, LA (Abstract/Poster # 2.285). https://www.aesnet.org/annual_meeting/abstract_search#/sortDate_na_dt/DESC/0/2.285/?Year=2018. Published to Web: 11/05/2018 07:00 pm.
- 16.Kasteleijn-Nolst Trenité DG, Reed RC. AEDs reduce the upper photosensitivity limit more than the lower photosensitivity limit in photosensitive patients: Implications for the design of the photosensitivity model. Epilepsy Curr. 2013;13(Suppl 1):1–492. [Google Scholar]
- 17.Kasteleijn-Nolst Trenité D, Rubboli G, Hirsch E, Martins da Silva A, Seri S, Wilkins A, et al. Methodology of photic stimulation revisited: updated European algorithm for visual stimulation in the EEG laboratory. Epilepsia. 2012;53(1):16–24. doi: 10.1111/j.1528-1167.2011.03319.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramael S, Daoust A, Otoul C, Toublanc N, Troenaru M, Lu ZS, et al. Levetiracetam intravenous infusion: a randomized, placebo-controlled safety and pharmacokinetic study. Epilepsia. 2006;47(7):1128–1135. doi: 10.1111/j.1528-1167.2006.00586.x. [DOI] [PubMed] [Google Scholar]
- 19.Abend NS, Monk HM, Licht DJ, Dlugos DJ. Intravenous levetiracetam in critically ill children with status epilepticus or acute repetitive seizures. Pediatr Crit Care Med. 2009;10(4):505–510. doi: 10.1097/PCC.0b013e3181a0e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berning S, Boesebeck F, van Baalen A, Kellinghaus C. Intravenous levetiracetam as treatment for status epilepticus. J Neurol. 2009;256:1634–1642. doi: 10.1007/s00415-009-5166-7. [DOI] [PubMed] [Google Scholar]
- 21.Nau KM, Divertie GD, Valentino AK, Freeman WD. Safety and efficacy of levetiracetam for critically ill patients with seizures. Neurocrit Care. 2009;11(1):34–37. doi: 10.1007/s12028-009-9185-0. [DOI] [PubMed] [Google Scholar]
- 22.Uges JW, van Huizen MD, Engelsman J, Wilms EB, Touw DJ, Peeters E, et al. Safety and pharmacokinetics of intravenous levetiracetam infusion as add-on in status epilepticus. Epilepsia. 2009;50(3):415–421. doi: 10.1111/j.1528-1167.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 23.Lyttle MD, Rainford NEA, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. 2019;393(10186):2125–2134. doi: 10.1016/S0140-6736(19)30724-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalziel SR, Borland ML, Furyk J, Bonisch M, Neutze J, Donath S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomized controlled trial. Lancet. 2019;393(10186):2135–2145. doi: 10.1016/S0140-6736(19)30722-6. [DOI] [PubMed] [Google Scholar]
- 25.Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, Shinnar S, Conwit R, Meinzer C, Cock H, Fountain N, Connor JT, Silbergleit R, for the NETT and PECARN Investigators Randomized trial of three anticonvulsant medications for status epilepticus. New Engl J Med. 2019;381:2103–2113. doi: 10.1056/NEJMoa1905795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sargentini-Maier ML, Rolan P, Connell J, Tytgat D, Jacobs T, Pigeolet E, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol. 2007;63(6):680–688. doi: 10.1111/j.1365-2125.2006.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasteleijn-Nolst Trenité DGA, Reed RC. The safety of augmented visual stimulation: does repeated, extensive visual stimulation within a time-frame of one hour increase sensitivity in photosensitive patients? Epilepsia. 2016;57(Suppl 2):86. [Google Scholar]
- 28.Luke SG. Evaluating significance in linear mixed-effects models in R. Behav Res Methods. 2017;49(4):1494–1502. doi: 10.3758/s13428-016-0809-y. [DOI] [PubMed] [Google Scholar]
- 29.Rheims S, Ryvlin P. Pharmacotherapy for tonic-clonic seizures. Expert Opin Pharmacother. 2014;15(10):1417–1426. doi: 10.1517/14656566.2014.915029. [DOI] [PubMed] [Google Scholar]
- 30.Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive SE: a meta-analysis of published studies. Seizure. 2014;23(3):167–174. doi: 10.1016/j.seizure.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Capovilla G, Beccaria F, Beghi E, Minicucci F, Sartori S, Vecchi M. Treatment of convulsive status epilepticus in childhood: recommendations of the Italian League Against Epilepsy. Epilepsia. 2013;54(Suppl 7):23–34. doi: 10.1111/epi.12307. [DOI] [PubMed] [Google Scholar]
- 32.Prasad M, Al-Roomi K, Krishnan PR, Sequeira R. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev. 2014;9:3723. doi: 10.1002/14651858.CD003723.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eue S, Grumbt M, Muller M, Schulze A. Two years’ experience in the treatment of status epilepticus with intravenous levetiracetam. Epilepsy Behav: E&B. 2009;15(4):467–469. doi: 10.1016/j.yebeh.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Langer JE, Fountain NB. A retrospective observational study of current treatment for generalized convulsive status epilepticus. Epilepsy Behav: E&B. 2014;37:95–99. doi: 10.1016/j.yebeh.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Strzelczyk A, Steinig I, Willems LM, Reif PS, Senft C, Voss M, et al. Treatment of refractory and super-refractory status epilepticus with brivaracetam: A cohort study from two German university hospitals. Epilepsy Behav: E&B. 2017;70(Pt A):177–181. doi: 10.1016/j.yebeh.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Strzelczyk A, Kay L, Bauer S, Immisch I, Klein KM, Knake S, et al. Use of brivaracetam in genetic generalized epilepsies and for acute, intravenous treatment of absence status epilepticus. Epilepsia. 2018;59(8):1549–1556. doi: 10.1111/epi.14476. [DOI] [PubMed] [Google Scholar]
- 37.Kalss G, Rohracher A, Leitinger M, Pilz G, Novak HF, Neuray C, et al. Intravenous brivaracetam in status epilepticus: A retrospective single-center study. Epilepsia. 2018;59(Suppl 2):228–233. doi: 10.1111/epi.14486. [DOI] [PubMed] [Google Scholar]
- 38.Aicua-Rapun I, Andre P, Rossetti A, Decosterd LA, Buclin T, Novy J. Intravenous brivaracetam in status epilepticus: Correlation between loading dose, plasma levels and clinical response. Epilepsy Res. 2019;149:88–91. doi: 10.1016/j.eplepsyres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Santamarina E, Parejo-Carbonell B, Sala J, Gutiérrez-Viedma Á, Miró J, Asensio M, et al. Use of intravenous brivaracetam in status epilepticus: A multicenter registry. Epilepsia. 2019;60(8):1593–1601. doi: 10.1111/epi.16094. [DOI] [PubMed] [Google Scholar]
- 40.Kwan P, Trinka E, Van Paesschen W, Rektor I, Johnson ME, Lu S. Adjunctive brivaracetam for uncontrolled focal and generalized epilepsies: results of a phase III, double-blind, randomized, placebo-controlled, flexible-dose trial. Epilepsia. 2014;55(1):38–46. doi: 10.1111/epi.12391. [DOI] [PubMed] [Google Scholar]
- 41.Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: Results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55(1):47–56. doi: 10.1111/epi.12432. [DOI] [PubMed] [Google Scholar]
- 42.KEPPRA® (levetiracetam) injection for intravenous use: US prescribing information. Smyrna, GA: UCB S.A., 2008.
- 43.BRIVIACT® (brivaracetam) tablets, for oral use, oral solution, injection for intravenous use: US presribing information. Smyrna, GA, UCB, Inc, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


