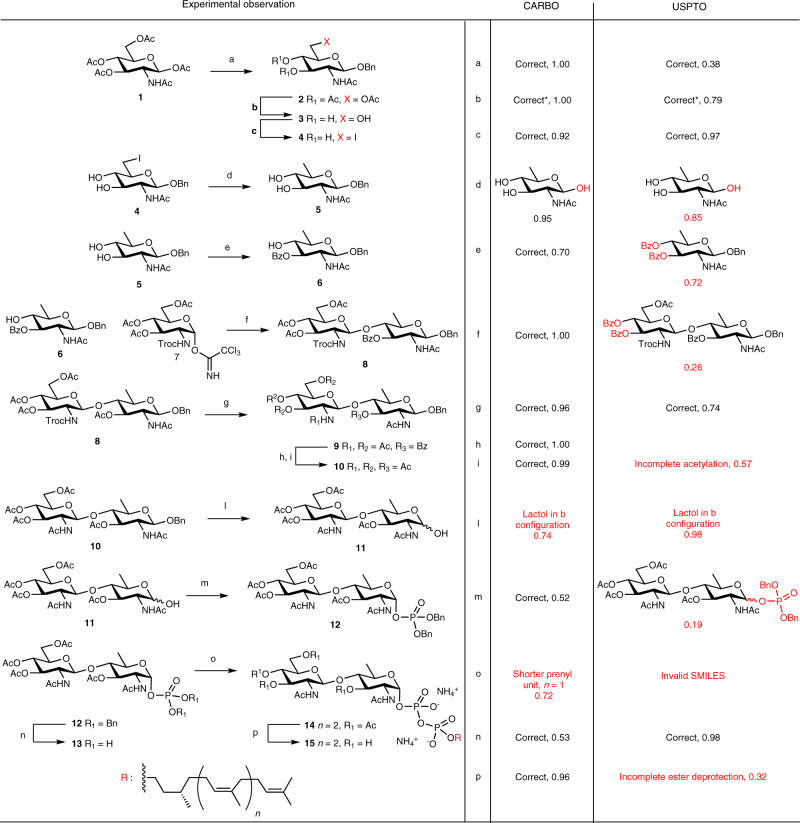
Fig. 4. Synthesis of lipid-linked oligosaccharide (LLO).
Reaction conditions: a BnOH, Yb(OTf)3, DCE, 90 °C, 2h, 78%. b MeONa, MeOH, sonication, 30 min. c PPh3, I2, imidazole, THF, 1h, reflux, 88% over two steps. d Pd/C, NH4OH, H2, THF/H2O, 30 min, 77%. e BzCl, pyr, −35°, 70%. f BF3Et2O, 4 MS, DCM, 26 h, 73%. g Zn, Ac2O, AcOH, DCE 50°, 3 h, 96%. h MeONa, MeOH/DMF, 4 days. i Ac2O, 4-(Dimethylamino)pyridine, pyr, 76% over three steps. l H2, THF/H2O, 10 bar, 16 h. m LiHMDS, tetrabenzylpyrophosphate, 53%. n H2, THF/MeOH, 1 h. o farnesylnerol, CDI, DMF, then 11, 5 days, 18%. p MeOH, NH4OH, 16 h, qte. An asterisk represents “*” reaction present in the training set.