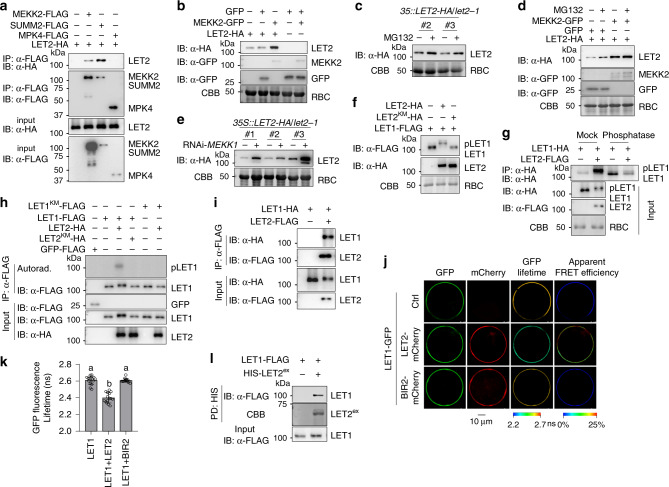
Fig. 4. LET1 and LET2/MDS1 regulation and heteromerization.
a LET2/MDS1 associates with MEKK2 and SUMM2, but not MPK4. LET2-HA was co-expressed with Ctrl, MEKK2-FLAG, SUMM2-FLAG, or MPK4-FLAG in protoplasts for 12 h. The FLAG-tagged proteins were immunoprecipitated by α-FLAG affinity beads, and then immunoblotted by an α-HA or α-FLAG antibody (top two panels). The proteins before immunoprecipitation were immunoblotted by an α-HA or α-FLAG antibody as inputs (bottom two panels). b MEKK2 stabilizes LET2/MDS1 protein accumulation in N. benthamiana. LET2-HA or GFP was co-expressed with MEKK2-GFP or GFP in N. benthamiana for 3 days. The proteins were immunoblotted by an α-HA or α-GFP antibody. CBB staining of RBC was used as a loading control. c MG132 treatment increases LET2/MDS1 protein accumulation in transgenic plants. The 10-day-old seedings of 35S::LET2-HA/let2-1 (Line #2 and #3) transgenic plants were treated with DMSO (Ctrl) or 5 μM MG132 for 6 h. Proteins were immunoblotted using an α-HA antibody, and CBB was used as a loading control. d MG132 treatment increases LET2/MDS1 protein accumulation in N. benthamiana. The leaves of N. benthamiana were inoculated with Agrobacterium carrying LET1-HA and GFP, or LET1-HA and MEKK2-GFP for 12 h, and then treated with DMSO (Ctrl) or 5 μM MG132 for anthor 36 h. Proteins were immunoblotted by an α-HA or α-GFP antibody. CBB was used as a loading control. e Silencing MEKK1 increases LET2/MDS1 protein accumulation. MEKK1 was silenced in the 35S::LET2-HA/let2-1 transgenic plants (Line #1, #2 and #3) by VIGS. Total proteins were extracted 2 weeks after VIGS, and immunoblotted using an α-HA antibody. CBB was used as a loading control. f LET2/MDS1, but not its kinase-inactive mutant LET2KM, induces LET1 mobility shift. LET2-HA or LET2KM-HA was co-expressed with LET1-FLAG in protoplasts for 12 h. LET1-FLAG was separated by 7.5% SDS-PAGE. CBB staining of RBC was used as a loading control. g LET2/MDS1 induces LET1 phosphorylation. LET1-HA was co-expressed with Ctrl or LET2-FLAG in protoplasts for 12 h. LET1-HA was immunoprecipitated by α-HA affinity beads. The immunoprecipitated LET1-HA protein was incubated without or with 0.5 μL (200 U) λ-phosphatase (Sigma) for 1 h at 30 °C. LET1-HA was separated by 10% SDS-PAGE and detected by an α-HA antibody (top panel). LET1-HA and LET2-FLAG before immunoprecipitation were detected by the corresponding antibody (middle two panels). CBB staining of RBC was used as a loading control (bottom panel). h LET2/MDS1 increases LET1 kinase activity. LET1-FLAG or LET1KM-FLAG was co-expressed with the vector control, LET2-HA or LET2KM-HA, in protoplasts. The FLAG-tagged proteins were immunoprecipitated from the cell lysates with α-FLAG affinity beads and used in a kinase assay with [γ-32P] ATP. The GFP-FLAG was used as a negative control. The proteins were immunoblotted by an α-FLAG or α-HA antibody for input controls. i LET1 associates with LET2/MDS1. LET1-HA was co-expressed with Ctrl or LET2-FLAG in protoplasts for 12 h. The LET2-FLAG proteins were immunoprecipitated by α-FLAG affinity beads, and then immunoblotted by an α-HA or α-FLAG antibody (top two panels). The proteins before immunoprecipitation were immunoblotted by an α-HA or α-FLAG antibody as inputs (bottom two panels). j, k FRET-FLIM analysis of LET1 and LET2/MDS1 interaction in Arabidopsis protoplasts. The indicated proteins were transiently expressed in protoplasts for 16 h, and FRET-FLIM was visualized using a confocal laser scanning microscopy (j). Localization of the LET1-GFP and LET2-mCherry/BIR2-mCherry is shown with the first (Green) and second column (Red), respectively. The lifetime (τ) distribution (third column), and apparent FRET efficiency (fourth column) are presented as pseudo-color images according to the scale. The GFP mean fluorescence lifetime (τ) values, ranging from 2.2 to 2.7 nanoseconds (ns), were statistically analyzed and are shown as mean ± SD (n = 15) (k). P = 1.07 × 10−12 (column 1 and 2), P = 1.08 × 10−12 (column 2 and 3). The different letters indicate the significant difference determined by one-way ANOVA followed by the Tukey test (P < 0.05). Scale bar, 10 µm. l LET2ex associates with LET1 in a pull-down assay. Arabidopsis protoplasts expressing LET1-FLAG were incubated with purified HIS-SUMO-LET2ex proteins. The interaction between LET1 and LET2ex was detected by an α-FLAG immunoblot after pull-down with Ni-NTA agarose. HIS-SUMO-LET2ex proteins were stained by CBB. The above experiments were repeated three times with similar results.