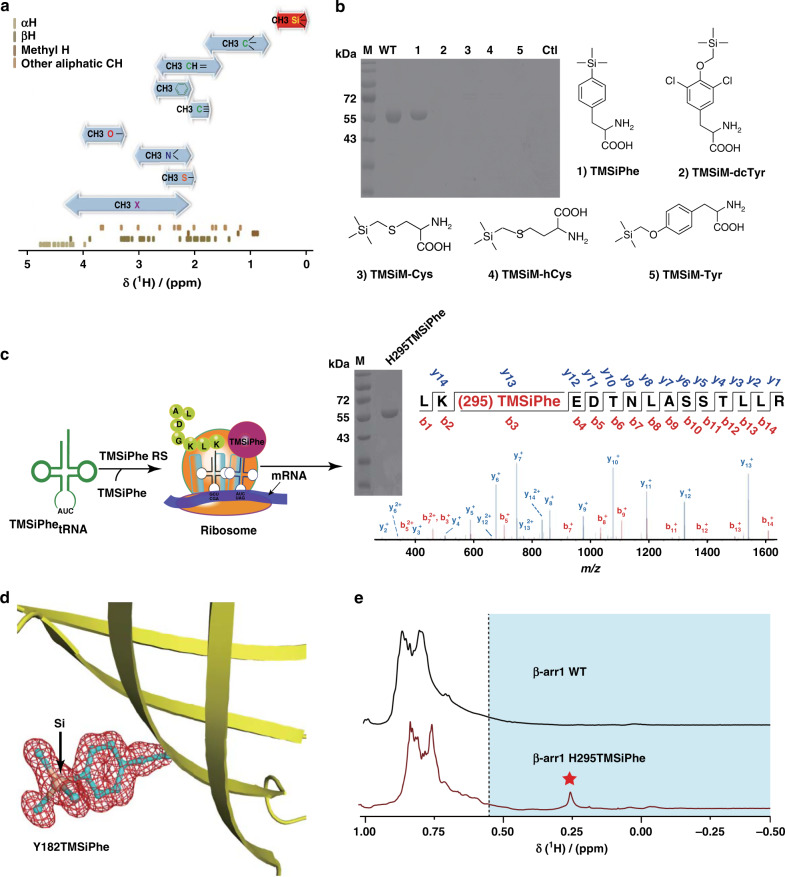
Fig. 1. Development of TMSiPheRS by genetic code expansion and the selectivity of TMSiPheRS.
a The ranges of the methyl 1H chemical shifts64 (shown by bidirectional arrows) and the distribution of random-coil aliphatic CH 1H chemical shifts for the 20 genetically coded amino acids65. The 1H chemical shifts of methyl silicon group are specified in red. b Coomassie-stained gel analysis of full-length β-arr1 expression in E. coli cells that were co-transformed with the β-arr1-H295 TAG plasmid and the pEVOL-TMSiPheRS plasmid in the presence or absence of different silicon-containing compounds. WT: β-arr1 wild-type. Full-length β-arr1 protein was obtained only in the presence of TMSiPhe for TAG mutation of β-arr1 or WT. These results suggested that the evolved TMSiPheRS exhibited significant structural selectivity for TMSiPhe over other silicon-containing chemicals. The chemical abbreviations are as follows: (1) 4-(trimethylsilyl) phenylalanine, TMSiPhe; (2) 3,5-dichloro-4-[(trimethylsilyl) methoxy]phenylalanine, TMSiM-dcTy; (3) 2-amino-3-((trimethylsilyl)methylthio)propanoic acid, TMSiM-Cys; (4) 2-amino-4-((trimethylsilyl)methylthio)butanoic acid, TMSiM-hCys; (5) 4-[(trimethylsilyl)ethoxy]phenylalanine, TMSiM-Tyr; (6), Ctl: control, no TMSiPhe added to the culture. c Schematic flowchart for the incorporation of TMSiPhe into β-arr1 at the H295 site. Full-length β-arr1 protein was obtained by co-transformation with the β-arr1-H295 TAG mutant plasmid and the pEVOL-TMSiPheRS plasmid, with TMSiPhe supplementation of the culture medium. The purity of the protein was determined by electrophoresis. The protein was subjected to trypsin digestion and analyzed by MS/MS. These results unambiguously confirmed that TMSiPhe was selectively incorporated into β-arr1 at the H295 position. m/z, mass/charge ratio. d The 2Fo-Fc annealing omit map of sfGFP Y182TMSiPhe clearly shows the electron density of TMSiPhe. The map was contoured at 1.1 σ. e 1D 1H NMR spectra for the β-arr1 H295TMSiPhe mutant were compared with β-arr1 WT cultured in the presence of TMSiPhe. The spectra were recorded in a buffer containing 50 mm Tris-HCl (pH = 7.5) and 150 mm NaCl at 25 °C using a Bruker 950 MHz NMR spectrometer. The β-arr1 H295TMSiPhe chemical shift at 0.26 ppm was consistent with the predicted chemical shift of the TMSi group. The 1H NMR signals of TMS group substituted amino acids in a protein were generally located in the high-field region (<0.55 ppm, blue area). Red pentagram: the position of NMR signal peak for the β-arr1-H295 TMSiPhe.