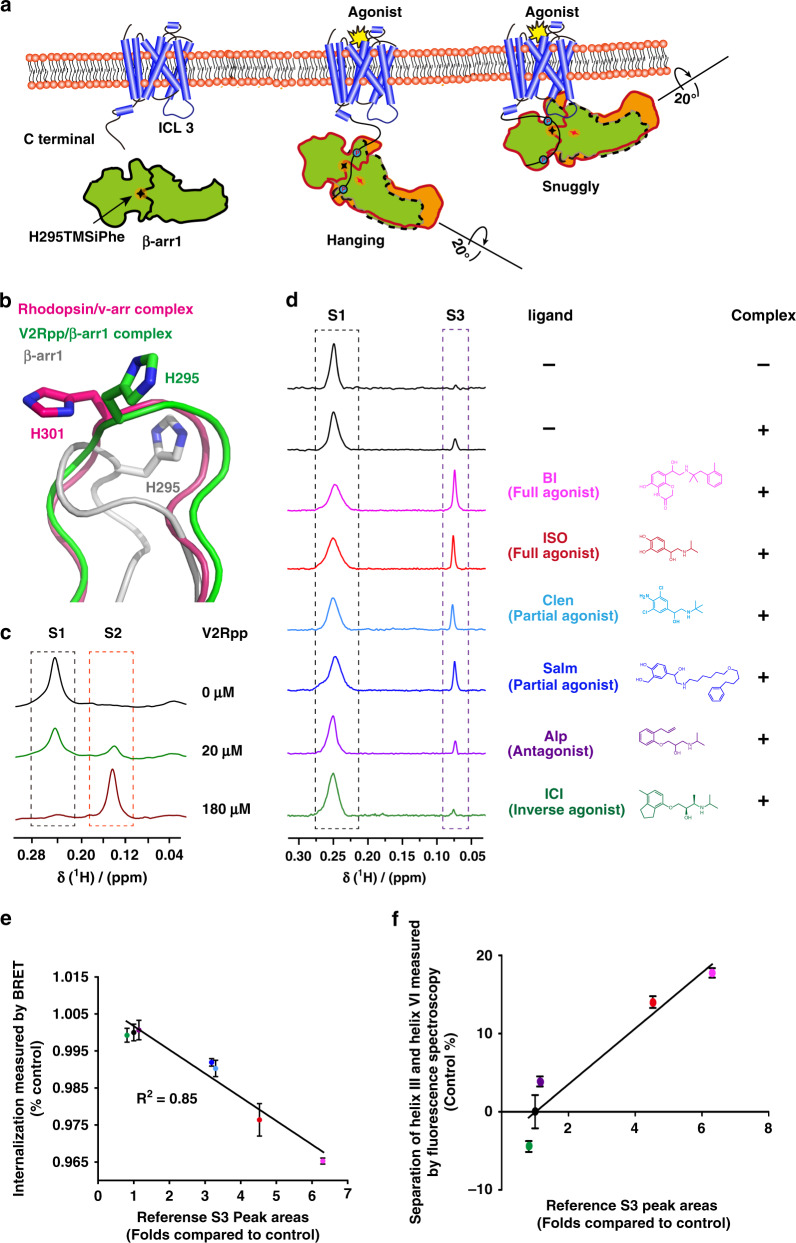
Fig. 4. Regulation of the β-arr1 polar core by different β2AR ligands.
a Cartoon illustration of two distinct interaction modes between GPCRs and β-arr1 (hanging mode and snug mode). The blue circles: phosphorylation. The Shuriken: the position of H295TMSiPhe in inactive (black) and active (red) β-arr1. b Structural comparison of the H295 position in inactive β-arr1 (PDB: 1G4M), the V2Rpp/ β-arr1 complex (PDB: 4JQI) and the rhodopsin/arrestin complex (PDB: 5W0P). The inactive β-arr1 structure is depicted in gray; the V2Rpp/β-arr1 complex is in green; and the rhodopsin-arrestin complex is in red. c 1D 1H NMR spectra of β-arr1-H295TMSiPhe in response to titration with V2Rpp. With increasing concentrations of V2Rpp, the peak at 0.25 ppm decreased (representing the S1 state), whereas a new growing peak was observed at 0.15 ppm (representing the S2 state). d 1D 1H NMR spectra of β-arr1 H295TMSiPhe alone or the ppβ2V2R/β-arr1 H295TMSiPhe/Fab30 complex with or without different ligands and the chemical structures of the ligands used in the current study. After incubation with the phospho-β2AR-V2-tail (ppβ2V2R) and formation of the receptor-arrestin complex, a new NMR signal appeared at 0.07 ppm (designated S3), and the intensity of the S1 peak decreased. When incubated with different β2AR ligands before formation of the ppβ2V2R-β-arr1/Fab30 complex, the S3 state signal intensity of the complex was positively correlated with effects of the ligands on the activation of downstream effectors, such as arrestin. BI BI-167107, ISO isoproterenol, Clen clenbuterol, salm salmeterol, Alp alprenolol; ICI ICI-118551. The buffer used for the experiment contained 20 mm HEPES, 150 mm NaCl, 0.01% LMNG, 0.002% CHS, and 10% D2O (pH = 7.5 at 25 °C). +: receptor-β-arr1 complex; β-arr1 alone. e Best-fit linear correlation of the peak area representing the amount of the S3 state in the presence of different ligands, with the ligand efficacy for receptor internalization from the BRET experiment in vivo. See Supplementary Fig. 22 for details. f Best-fit linear correlation of the peak area representing the amount of the S3 state in the presence of different ligands, with the ligand efficacy for separation of the receptor transmembrane III and VI from the TRIQ experiment in vitro63. See Supplementary Fig. 23 for details.