Figure 3.
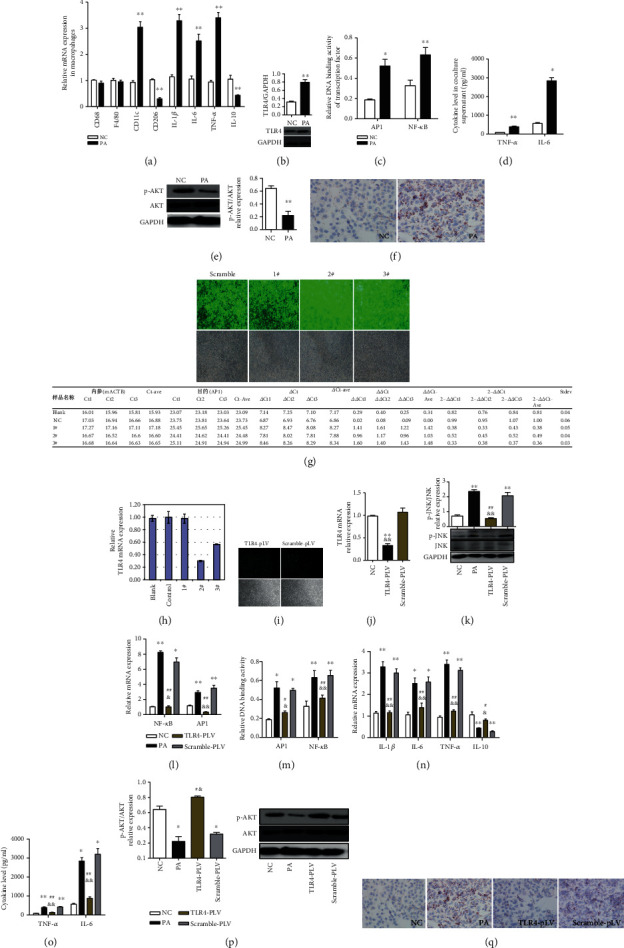
TLR4 siRNA transfection mitigated PA-induced inflammation in RAW264.7 cells and metabolic abnormalities in cocultured AML hepatocytes. The expressions of proinflammatory macrophage (M1 type) specific surface marker CD11c and proinflammatory factors (IL-1β, IL-6, and TNF-α) were significantly increased, while the expressions of anti-inflammatory macrophage (M2 type) specific surface marker CD206 and anti-inflammatory factor IL-10 were obviously decreased in palmitic acid- (PA-) treated RAW264.7 cells (a). PA treatment promoted the expression of TLR4 mRNA and protein and Ikkβ/NF-κB, activation of JNK/AP1 pathway in RAW264.7 cells, and production of proinflammatory factors (TNF-α and IL-6) in the supernatant (b–d). The phosphorylation of AKT was significantly decreased, and intracellular lipid accumulation was significantly increased in cocultured AML12 hepatocytes (e, f). TLR4 transfection significantly downregulated the TLR4 mRNA expression in RAW264.7 macrophages (g, j). TLR4 siRNA transfection mitigated PA-induced augmentation in JNK phosphorylation (k). TLR4 siRNA transfection could attenuate PA-induced increases in the mRNA expression and DNA binding activity of AP1 and NF-κB (l, m), as well as proinflammatory factor (IL-1β, IL-6, and TNF-α) expression in RAW264.7 macrophages and TNF-α and IL-6 levels in the supernatant (n, o). TLR4 siRNA transfection alleviated PA-induced decreases in the mRNA expression of IL-10 (n). TLR4 siRNA transfection in RAW264.7 macrophages significantly ameliorated the decreases in AKT phosphorylation and lipid accumulation in cocultured AML12 hepatocytes (p, q). Data are presented as mean ± SEM; ∗p < 0.05 and ∗∗p < 0.01 compared with the NC group; &p < 0.05 and &&p < 0.01 compared with the Scramble-pLV group (j–n); #p < 0.05 and ##p < 0.01 compared with the PA group (k–n).
