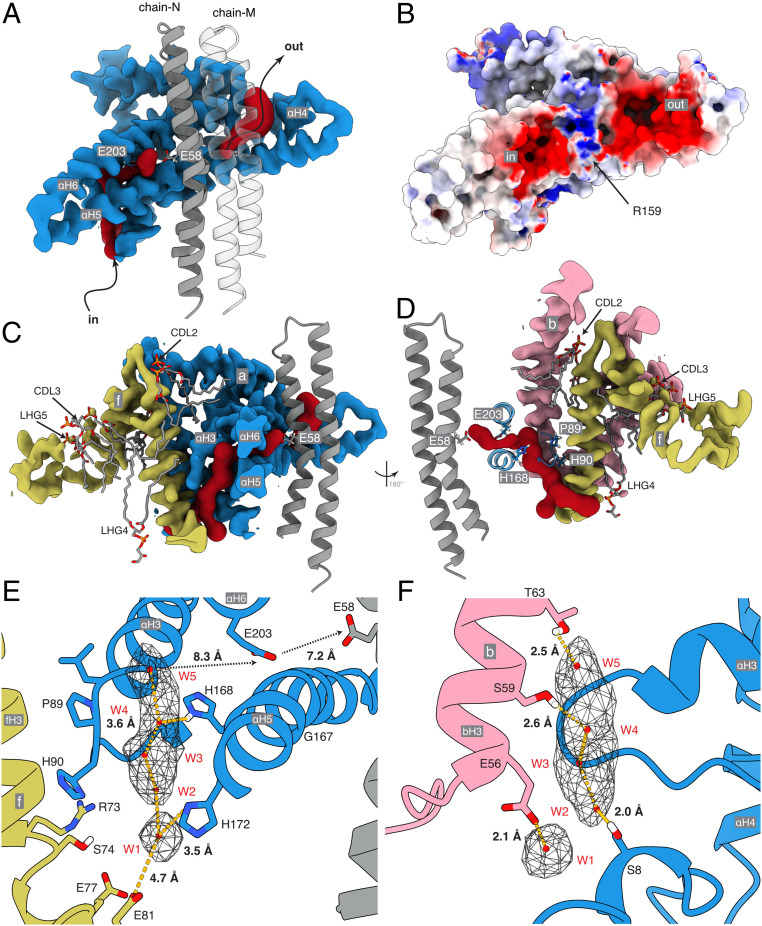
Fig. 2.
Proton half-channels in bovine ATP synthase with a Grotthus water chain in the inlet. (A and B) Cross-section of part of the a-subunit (blue) plus a two adjacent c-subunits (gray) viewed from inside the c8-ring. (A) The inverted L-shape of the inlet channel and the tilted outlet half-channel (both red) determined with CAVER. (B) The electrostatic potential surface of the inlet and outlet half-channels separated by a region of positive charge around aArg-159. The electrostatic potential of the molecular surface, calculated with the DELPHIpKa web-server (62, 63) at a salt concentration of 150 mM, a pH of 7.4, and otherwise default parameters, is shown with positively and negatively and positively charged surfaces in blue and red, respectively, and the c8-ring in transparency. The range of charge potentials is −5 (red) to +7 (blue). (C and D) The topology of the inlet cavity with a vertical cross-section of the membrane domain of the proteins and adjacent phospholipids CDL2, CDL3, LHG4, and LHG5. (E and F) Detailed views of the inlet channel with charged and polar residues available for hydrogen bonding a Grotthuss chain of five water molecules W1 to W5 (red spheres) suggested by the unmodeled density (in mesh). The view in E is equivalent to that in C. F is viewed through the channel from cGlu-58 toward the loop of subunit a containing aPro-90 (D). Distances between H-bond donors and acceptors and water molecules are shown. Between W1 and W5 the distances are 4.1, 3.2, 2.6, and 4.0 Å, respectively. The Grotthuss chain leads to aGlu-203 and then to cGlu-58 possibly via other as-yet-undefined water molecules.