Significance
Uterine glands play a critical role in embryo implantation and pregnancy establishment. Using mouse genetic models, we found that FOXA2-regulated genes in the uterine glands influence development of the decidua, as well as the placenta and fetus in a sex-dependent manner. These findings support the idea that uterine gland dysfunction affects placental and fetal growth with sexually dimorphic impacts on offspring adult health and disease.
Keywords: uterus, gland, FOXA2, pregnancy, placenta
Abstract
Glands of the uterus are essential for pregnancy establishment. Forkhead box A2 (FOXA2) is expressed specifically in the glands of the uterus and a critical regulator of glandular epithelium (GE) differentiation, development, and function. Mice with a conditional deletion of FOXA2 in the adult uterus, created using the lactotransferrin iCre (Ltf-iCre) model, have a morphologically normal uterus with glands, but lack FOXA2-dependent GE-expressed genes, such as leukemia inhibitory factor (LIF). Adult FOXA2 conditional knockout (cKO; LtfiCre/+Foxa2f/f) mice are infertile due to defective embryo implantation arising from a lack of LIF, a critical implantation factor of uterine gland origin. However, intraperitoneal injections of LIF can initiate embryo implantation in the uterus of adult FOXA2 cKO mice with pregnancies maintained to term. Here, we tested the hypothesis that FOXA2-regulated genes in the uterine glands impact development of the decidua, placenta, and fetus. On gestational day 8.5, the antimesometrial and mesometrial decidua transcriptome was noticeably altered in LIF-replaced FOXA2 cKO mice. Viable fetuses were reduced in FOXA2 cKO mice on gestational days 12.5 and 17.5. Sex-dependent differences in fetal weight, placenta histoarchitecture, and the placenta and metrial gland transcriptome were observed between control and FOXA2 cKO mice. The transcriptome of the placenta with a female fetus was considerably more altered than the placenta with a male fetus in FOXA2 cKO dams. These studies reveal previously unrecognized sexually dimorphic effects of FOXA2 and uterine glands on fetoplacental development with potential impacts on offspring health into adulthood.
In mice, the initiation of embryo attachment and implantation, occurring late on gestational day (GD) 4, is followed by extensive proliferation of stromal cells surrounding the nidating blastocyst on the morning of GD 5 (1–3). Between GDs 5 and 6, stromal cells, adjacent to the implanted blastocyst in the antimesometrial (AM) region of the uterus, cease proliferating and undergo differentiation into decidual cells, forming the primary decidual zone (PDZ), an area that is avascular and epithelioid in nature (4). Stromal cells adjacent to the PDZ continue to proliferate and differentiate into polyploid cells forming the secondary decidual zone (SDZ), which is located in the AM region of the uterus and fully developed by GD 6.5 (5). Decidualization then progresses in the mesometrial (M) region of the uterus, forming the decidua basalis (DB) (6). The DB is adjacent to the site of placental development from the ectoplacental cone (7). Placental development involves proliferation and outgrowth of trophectoderm cells, formation of the allantois and its fusion with the chorion on GD 8.5, and formation of the placental labyrinth zone (8). Placental development is essentially complete by GD 11.5 in mice. Impairment or alterations in stromal cell decidualization frequently results in embryo implantation failure, placental failure, pregnancy complications, or miscarriage in mice and humans (8–14). Uterine glands secrete proteins and other factors apically into the uterine lumen and basally toward the stroma/decidua (15), and recent evidence indicates that uterine glands influence stromal cell decidualization (15–18). Additionally, uterine glands have direct connections to the developing placenta in mice (19, 20) and humans (21).
Mice lacking leukemia inhibitory factor (LIF) and uterine gland knockout mice and sheep are infertile, thereby establishing the importance of the uterine glands, their secretions, and products for embryo implantation and pregnancy (22–28). Forkhead box (FOX) transcription factors play essential roles in cell growth, proliferation, and differentiation in a number of different organs, including the uterus (29, 30). In the uterus, FOXA2 is expressed specifically in the glandular epithelium (GE) of the uterine endometrium in neonatal and adult mice (24, 25, 31), as well as humans (32). To interrogate the role of Foxa2 in the uterus, it was conditionally deleted in the uterus of neonatal mice using the progesterone receptor Cre (PgrCre) mouse (33). The uterus of those FOXA2 conditional knockout (cKO) mice lacked GE differentiation, resulting in defects in embryo attachment for implantation, and thus infertility (24, 25). Of note, those FOXA2 cKO mice lacked expression of LIF that is specifically induced by ovarian estrogen in the GE on GD 4 (34, 35). LIF is critical for embryo implantation (28, 35) and has pleiotropic effects on the uterine epithelium to regulate uterine receptivity (34, 36). In addition, FOXA2 has been conditionally deleted in the adult uterus using the lactotransferrin iCre (LtfiCre) mouse model (37). Although the uterus of adult FOXA2 cKO mice (LtfiCre/+Foxa2f/f) was histologically normal with glands, embryo implantation defects were observed and, unexpectedly, those mice lacked nidatory Lif expression (18). Intraperitoneal injections of recombinant mouse LIF initiated embryo implantation in both types of FOXA2 cKO mice (18). Pregnancy was maintained to term in gland-containing adult FOXA2 cKO (LtfiCre/+Foxa2f/f) mice, but failed by GD 10 in glandless neonatal FOXA2 cKO (PgrCre/+Foxa2f/f) mice lacking uterine glands. Although pregnancy did not fail in LIF-replaced adult FOXA2 cKO mice (17, 18), genes in the decidua were altered on GDs 6 and 10 (18) and in the placenta and fetal brain on GD 15 (38). In addition to Lif, a number of progesterone-induced genes expressed in the uterine glands (e.g., Prss28, Prss29, Spink1) were not expressed in the uterus of adult FOXA2 cKO mice. Genomic studies have identified several genes that are dependent on, or regulated by, FOXA2 in the glands of the mouse and human uterus (16–18, 31, 39, 40). In other tissues and organs, FOXA factors and their targets display sexual dimorphism (41, 42). Indeed, clear sex-specific differences exist in fetoplacental development and response of the placenta and fetus to maternal and environmental stressors (9, 43, 44).
Collective in vivo evidence supports the hypothesis that FOXA2 regulates Lif expression in glands of the mouse uterus for embryo implantation and that FOXA2-independent and -dependent factors from uterine glands play an active role in postimplantation stromal cell decidualization critical for placental development and pregnancy establishment. Here, we continue to address that hypothesis by studying LIF-replaced adult uterine FOXA2 cKO dams and focusing on postimplantation placental and fetal development with consideration of sexual dimorphism.
Results
Pregnancy Outcomes in LIF-Replaced Uterine FOXA2 cKO Uteri.
For these studies, littermate control (Foxa2f/f) and FOXA2 cKO (LtfiCre/+Foxa2f/f) adult mice were bred to males of proven fertility. On GD 4, FOXA2 cKO mice received LIF injections to initiate embryo implantation. As summarized in Table 1, the number of viable fetuses was lower by ∼1.7 or 1.2 pups in FOXA2 cKO dams on GDs 12.5 and 17.5, respectively, and nonviable fetuses were greater in FOXA2 cKO dams on GD 17.5. As reported previously (18), LIF-replaced FOXA2 cKO mice gave birth to live young with no difference in gestation length or litter size compared to control mice.
Table 1.
Characteristics of the fetus and placenta in littermate control and FOXA2 cKO dams
| GD 12.5 | GD 17.5 | |||||
| Parameter | Control | FOXA2 cKO | P value | Control | FOXA2 cKO | P value |
| Fetus number | ||||||
| Viable | 9.0 ± 0.3 (10) | 7.3 ± 0.5 (8) | 0.03 | 9.5 ± 0.4 (13) | 8.3 ± 0.4 (12) | 0.03 |
| Nonviable | 0.2 ± 0.1 (10) | 0.9 ± 0.4 (8) | 0.18 | 0.5 ± 0.2 (13) | 1.7 ± 0.5 (12) | 0.05 |
| Fetal Weight (mg) | ||||||
| Male fetus | 98.5 ± 2.8 (28) | 84.8 ± 2.8 (20) | <0.01 | 1046.8 ± 13.8 (35) | 937.2 ± 13.9 (42) | <0.01 |
| Female fetus | 100.0 ± 2.6 (21) | 77.8 ± 2.7 (12) | <0.01 | 973.6 ± 19.0* (34) | 937.8 ± 14.9 (30) | 0.14 |
| Placenta weight (mg) | ||||||
| Male fetus | 54.9 ± 1.8 (28) | 53.0 ± 2.1 (20) | 0.88 | 114.8 ± 2.4 (35) | 123.8 ± 1.7 (42) | <0.01 |
| Female fetus | 56.6 ± 1.8 (21) | 48.0 ± 1.3* (12) | 0.01 | 108.7 ± 1.8* (34) | 119.2 ± 2.2* (30) | <0.01 |
| Fetus:placenta weight ratio | ||||||
| Male fetus | 1.81 ± 0.03 (28) | 1.58 ± 0.03 (20) | <0.01 | 9.24 ± 0.20 (35) | 7.62 ± 0.15 (42) | <0.01 |
| Female fetus | 1.78 ± 0.04 (21) | 1.63 ± 0.06* (12) | 0.08 | 9.01 ± 0.20 (34) | 7.93 ± 0.17 (30) | <0.01 |
| Trophoblast invasion index | ||||||
| Male fetus | 0.18 ± 0.05 (6) | 0.28 ± 0.02 (6) | 0.03 | 0.64 ± 0.06 (6) | 0.60 ± 0.02 (6) | 0.37 |
| Female fetus | 0.28 ± 0.03* (6) | 0.23 ± 0.05 (6) | 0.72 | 0.60 ± 0.05 (6) | 0.51 ± 0.04 (6) | 0.19 |
| Junctional zone (percent of cells in placenta) | ||||||
| Male fetus | 34.5 ± 0.5 (6) | 39.1 ± 1.7 (6) | 0.06 | 26.4 ± 1.8 (6) | 26.2 ± 1.7 (6) | 0.39 |
| Female fetus | 32.0 ± 1.2 (6) | 35.7 ± 1.3 (6) | 0.04 | 22.4 ± 1.1 (6) | 32.0 ± 1.9 (6) | <0.01 |
Measurements are provided as mean with SE and number of observations (n) per genotype evaluated.
Effect of sex within genotype (male versus female, P < 0.05) on the parameter.
Effects of Uterine FOXA2 Deletion on the Transcriptome of the AM and M Decidua.
Bred control and FOXA2 cKO dams were necropsied on GD 8.5. Implantation sites were carefully opened, and the embryo and ectoplacental cone removed for sex determination by PCR genotyping. The decidua from the AM and M regions were then physically separated and evaluated by RNA sequencing (RNA-seq) (Fig. 1 and Dataset S1).
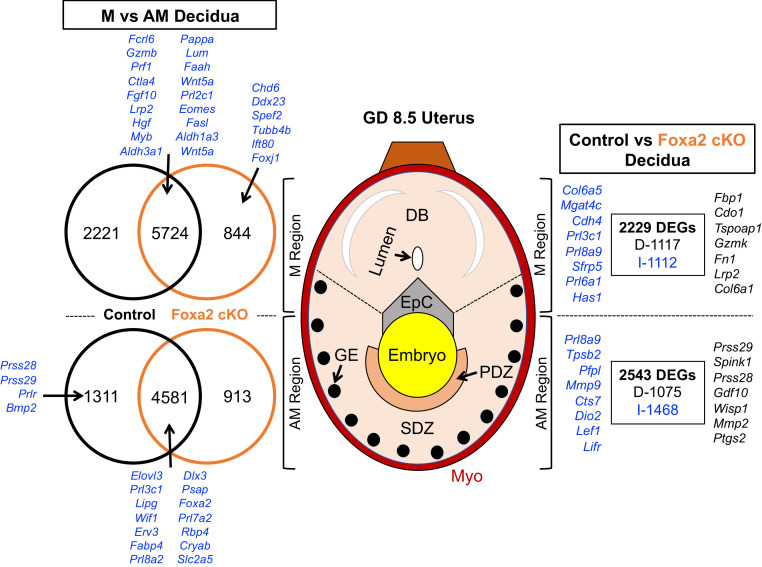
Fig. 1.
Effects of fetal sex and uterine FOXA2 deletion on the GD 8.5 uterus transcriptome. The M and AM areas of the uterus were isolated, and RNA-seq analysis was conducted using tissues from control and LIF-replaced FOXA2 cKO dams (n = 5 per sex and genotype). DEGs (FDR P < 0.05) for comparisons are presented as decreased (D; black) or increased (I; blue) in Venn diagrams. EpC, ectoplacental cone; Myo, myometrium.
Control Dams.
As expected, substantial differences in gene expression (13,837 differentially expressed genes, DEGs) were detected (false-discovery rate [FDR] P ≤ 0.05) between the M and AM decidua in control dams. In contrast, there were few sex-specific differences in the transcriptome of either the M or AM decidua. Only four genes were more abundant (Ddx3g, Eif2s3y, Kdm5d, Uty) in the AM decidua with a male than female embryo. In the M decidua, only 28 DEGs were found, with 6 higher in the M decidua with a male embryo (Eif2s3y, Ddx3y, Rn7sk, Gm23935, Lars2, CT010467.1) and 21 higher with a female embryo (Hba-a1, Hbb-bs, Fabp4, Calml3, Psg22, Gm15772, Hbb-bt, Chrdl2, H19, Nppc, Osm, Rhox9, Mir351, Rhox6, Gm9513, Gm7257, Ly6k, Ighg2b, Hbb-y, Hba-x, Hbb-bh1). Given the relative lack of embryo sex-dependent effects on the decidual transcriptome, RNA-seq data were pooled across sex and reanalyzed.
In the AM decidua containing the PDZ and SDZ, many genes (5,892) were more abundant than in the M uterus, with many of those encoding genes specifically expressed in the GE (Prss28, Prss29, Spink1, Foxa2) or in the cells of the PDZ and SDZ (Prl8a2, Bmp2, Wnt4). As detailed in Dataset S1, gene ontology (GO) enrichment and biological pathway analysis, based on hypergeometric distribution followed by FDR correction (45), revealed that genes (fold-change ≥ 2) more abundant in the AM than M decidua were enriched in biological process (e.g., ceramide metabolic process, sphingolipid catabolic process). Analysis of the upstream 600 bp of those genes found enrichment in specific transcription factor motifs that included members of the AP-2 family (Tcfap2a, Tcfap2b, Tcfap2c, Tcfap2e), bHLH family (Hes1, Hes7), and C2H2 ZF family (e.g., Sp1, Egr1).
In the M decidua containing the DB, many genes (7,945) were more abundant, including those expressed in specific immune cell populations, such as uterine natural killer cells (Il15) and cytotoxic T lymphocytes (Gzmb, Prf1), that are particularly abundant in the DB (46). As summarized in Dataset S1, genes more abundant in the M decidua were enriched in GO terms associated with biological process (e.g., immune response, biological adhesion, cell activation, cytokine production), cellular component (e.g., cell surface, extracellular matrix, extracellular region, basement membrane), molecular function (e.g., extracellular matrix structural constituent, carbohydrate binding, cytokine receptor activity, hormone binding), as well as Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (e.g., natural killer cell-mediated cytotoxicity, cell adhesion molecules, cytokine–cytokine receptor interaction, extracellular matrix–receptor interaction). Analysis of the upstream 600 bp of those genes found enrichment in specific transcription factor motifs that included members of the C2H2 ZF family (e.g., Glis2, Egr1, Klf5, Sp2), EF2 family (E2f3), and bHLH family (Tcf3).
FOXA2 cKO Dams.
Substantial differences (12,062 DEGs) in the M and AM decidua transcriptome were also identified in FOXA2 cKO dams. Compared to control dams, there were common and unique differences in the transcriptome of genes more abundant in the AM decidua. Analysis of the 885 unique genes increased in the AM decidua of FOXA2 cKO dams revealed no enriched GO terms or biological pathways. In contrast, the 1,292 unique genes increased in the AM decidua were enriched in biological process (ubiquitin-dependent protein catabolic process, cellular protein catabolic process) and a biological pathway (endocytosis). The 819 unique genes increased in the M decidua of FOXA2 cKO dams were not enriched in GO terms or biological pathways.
AM Decidua.
Comparison of control and FOXA2 cKO dams revealed 2,543 DEGs in the AM decidua (Fig. 1). The 1,075 genes less abundant in the FOXA2 cKO AM decidua included established FOXA2-dependent GE-specific genes (Spink1, Prss29, Prss28) as well as genes expressed in the PDZ (Ptgs2). The 1,468 genes more abundant in the FOXA2 cKO AM decidua included genes known to be expressed in the decidualized stromal cells (Lef1, Lifr, Mmp9), including members of the expanded prolactin (PRL) family (Prl8a9, Prl5a1, Prl3d3, Prl3d2). As summarized in Dataset S1, the DEGs were enriched in GO terms associated with cellular component (extracellular space).
M Decidua.
Comparison of control and FOXA2 cKO dams revealed 2,229 DEGs in the M decidua. The 1,117 genes decreased in the FOXA2 cKO uterus included those expressed in cytotoxic T cells (Gzmk). The 1,112 genes increased in the FOXA2 cKO M decidua included those known to be expressed by DB cells (Prl3c1, Prl8a9, Sfrp5, Prl6a1). The DEGs were not enriched in any GO terms or KEGG pathways.
Effects of FOXA2 Deletion on Development of the Placenta and Fetus.
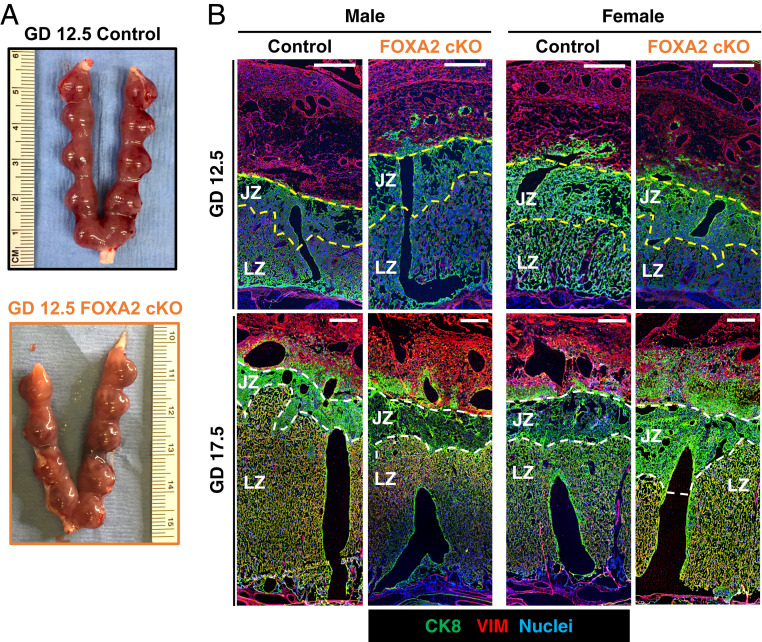
Placenta differentiation and development is essentially complete by GD 12.5 (8). The placentae of female fetuses, but not male fetuses, in FOXA2 cKO dams were ∼15% smaller in weight as compared to the placentae associated with female fetuses in control dams on GD 12.5 (Table 1). Interestingly, both male and female fetuses weighed less in FOXA2 cKO as compared to control dams on GD 12.5. Fetus-to-placenta weight ratios were lower for males in FOXA2 cKO dams. Histomorphometrical analyses were used to determine junctional zone (JZ) area and trophoblast invasion index, which measures the migration of trophoblast cells from the JZ into the DB (Fig. 2 and Table 1). The JZ area was greater in female, but not male, placentae from FOXA2 cKO dams. The trophoblast invasion index was not different in the placentae of control and FOXA2 cKO dams, regardless of fetal sex.
Fig. 2.
Placental development in control and LIF-replaced adult uterine FOXA2 cKO dams. (A) Embryo implantation sites in GD 12.5 control and FOXA2 cKO dams. (B) Immunolocalization of the pan trophoblast marker cytokeratin 8 (CK8), vimentin (VIM) in cross-sections of GDs 12.5 and 17.5 placenta. (Scale bars, 250 μm.)
In both control and FOXA2 cKO dams, female placentae weight was lower than male placentae on GD 17.5 (Table 1). Of note, the placentae of both male and female fetuses were heavier in FOXA2 cKO than control dams. The weight of male fetuses, but not female fetuses, in FOXA2 cKO dams were about 10% smaller than control dams. Fetal-to-placental weight ratios were lower for both sexes in FOXA2 cKO as compared to control dams. Area of the JZ was greater in female placentae in FOXA2 cKO than control dams, but not different in placentae with male fetuses. The trophoblast invasion index was not different between genotypes regardless of embryo sex.
Effects of Uterine FOXA2 Deletion on the Placenta and Metrial Gland Transcriptome.
Littermate control and LIF-replaced FOXA2 cKO dams were collected on either GD 12.5 or GD 17.5. Sex of the fetus from each placenta or metrial gland (MG) was determined by PCR. The transcriptome of the placenta and MG from male and female fetuses were interrogated by RNA-seq (Fig. 3 and Datasets S2–S4).
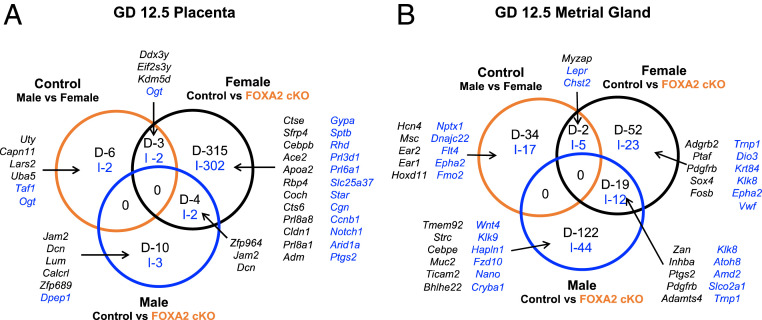
Fig. 3.
Effects of fetal sex and uterine FOXA2 deletion on the GD 12.5 placenta (A) and metrial gland (B) transcriptome. RNA-seq analysis was conducted using tissues from control and LIF-replaced FOXA2 cKO dams (n = 5 per sex and genotype). DEGs (FDR P < 0.05) for comparisons are presented as decreased (D; black) or increased (I; blue) in Venn diagrams.
GD 12.5 Placenta.
In control dams, few differences in gene expression (13 DEGs) were observed between male and female GD 12.5 placentae (Fig. 3A). Transcriptome analysis of GD 12.5 placentae from control and FOXA2 cKO dams revealed only 19 DEGs in the male placentae, whereas 628 DEGs were found in the female placentae. Expression of several expanded PRL family genes were either decreased (Prl8a6, Prl8a1, Prl8a8) or increased (Prl3d1, Prl6a1) in the female placenta of FOXA2 cKO dams. Several of the DEGs in the female placentae encoded factors known to regulate placental development (Adm, Arid1a, Bmp7, Bptf, Cebpb, E2f7, Egln1, Grhl2, Map3k4, Met, Ncoa6, Notch2, Pkd1, Plk4, Ptgs2, Spint2, Tfeb) based on the placenta development GO term (GO:0001890). Furthermore, some of the DEGs in the female placenta were imprinted (Dcn, Lin28a, Qpct, Slc22a18, Tfpi2, Tnfrsf23, Tspan32, Zdbf2) or located on the X-chromosome (Ace2, Amer1, Atp7a, Atrx, Bcor, Cstf2, Elf4, Fam90a1b, Gm21887, Hcfc1, Kif4, Maoa, Med12, Pet2, Pola1, Ptchd1, S100g, Sat1, Vgll1, Wdr45, Wnk3).
As summarized in Dataset S2, enrichment analysis found that the DEGs in the female placenta of control and FOXA2 cKO dams were associated with biological process (animal organ development, system development, mitotic cell cycle), cellular component (extracellular region, nonmembrane-bounded organelle, nuclear part, cell surface), molecular function (enzyme binding, small molecule binding, carbohydrate derivative binding), and KEGG pathways (cell cycle, cellular senescence, TGF-β signaling pathway, cell adhesion molecules). Of note, genes involved in reproductive structure development and reproductive system development (Cebpb, Atrx, Spp1, Inhba, Adam15, Srd5a1, Dcn, Grhl2, Ptgs2, Itgb8, Adm, Hoxa11, Gja1, Mgst1, Tfeb, Rbp4, Tgfb2, Spint2, Akap9, Plekha1) were decreased in the female placenta from FOXA2 cKO dams.
The genes decreased in the female placenta of FOXA2 cKO dams were enriched in biological process (response to bacterium, low-density lipoprotein particle remodeling), cellular component (extracellular region, extracellular space), and molecular function (purigenic nucleotide receptor activity, cholesterol transporter activity, lipid transporter activity) (Dataset S2). The genes increased in the female placenta of FOXA2 cKO dams were enriched in cellular component (spectrin-associated cytoskeleton), molecular function (NADH dehydrogenase activity), and KEGG pathways (oxidative phosphorylation, retrograde endocannabinoid signaling).
As expected, no enrichment for GO terms or biological pathways were found in the 19 DEGs in the male placenta between control and FOXA2 cKO dams or in the 13 DEGs between male and female placenta of control dams. Comparison of DEG in placenta from GDs 12.5 and 17.5 found only 18 (e.g., Serpinb9d, Prl8a1, Star, Slc39a3, Zdbf2, Zfhx3) and 1 (Calcrl) common genes in female and male pregnancies, respectively.
GD 12.5 MG.
The MG is a structure located in the M region of the uterus that forms around GD 8 (Fig. 3B and SI Appendix, Fig. S1). It is composed of a dynamic mixed-cell population of granulated immune cells, endometrial stromal cells, trophoblasts, and endothelial cells (47, 48). Sex-specific differences in the MG transcriptome were observed in control and FOXA2 cKO dams, but only a few genes were commonly different based on fetal sex and genotype.
Comparison of MG from control and FOXA2 cKO dams revealed differences with a female fetus (113 DEGs) or male fetus (197 DEGs), with only 31 common DEGs. The DEGs in the MG associated with the female fetus were enriched for GO terms for biological process (regulation of calcium-transporting ATPase activity, regulation of transporter activity), cellular component (anchored component of membrane), and molecular function (signaling pattern recognition receptor activity), but no KEGG pathways (Dataset S3). The DEGs in the MG associated with the male fetus were enriched in GO terms associated with molecular function (serine-type endopeptidase activity) and KEGG pathways (pertussis).
GD 17.5 Placenta.
In control dams, 90 DEGs were found in the placenta of male as compared to female fetuses. Enrichment analysis found that those DEGs were enriched for biological process (central nervous system development) (Fig. 4 and Dataset S4).
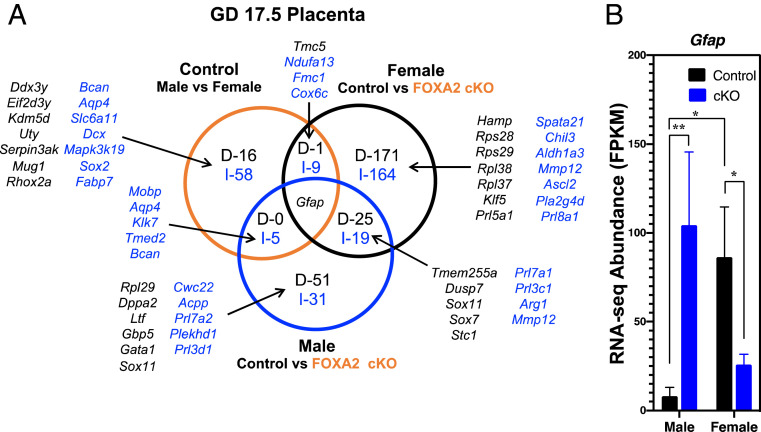
Fig. 4.
Effects of fetal sex and uterine FOXA2 deletion on the GD 17.5 placenta transcriptome. RNA-seq analysis was conducted using tissues from control and LIF-replaced FOXA2 cKO dams (n = 5 per sex and genotype). (A) DEGs (FDR P < 0.05) for comparisons are presented as decreased (D; black) or increased (I; blue) in Venn diagrams. (B) Abundance of Gfap mRNA based on RNA-seq analysis of the placentae from control and FOXA2 cKO dams.
Placentae with female fetuses had more gene-expression differences than the male placenta (390 vs. 132 DEGs, respectively) between control and FOXA2 cKO dams. The intersection of those data revealed 44 common DEGs (19 increased, 25 decreased) in the placentae of FOXA2 cKO dams regardless of sex. Of note, Gfap was differentially altered between the two sexes in control as compared to FOXA2 cKO dams (Fig. 4B). The 45 common DEGs were not enriched for any GO terms or biological pathways.
For the female placenta, the DEGs were enriched for GO terms involved in biological process (killing of cells of other organism, disruption of cells of other organism), cellular component (cytoplasmic side of rough endoplasmic reticulum membrane), and molecular function (e.g., neurotransmitter binding, calcium ion binding, carbohydrate binding, receptor ligand activity, prolactin receptor binding) (Dataset S4). Of note, the DEGs encoded a number of transcription factors (Cdx2, Hey1, Mxi1, Per2, Sox4, Sox7, Sox11, Sox17, Tbx3), ribosomal subunits (Rpl18a, Rpl21, Rpl27, Rpl32, Rpl35, Rpl36a, Rpl37, Rpl38, Rpl41, Rps17, Rps19bp1, Rps23, Rps27l, Rps28, Rps29), and members of the expanded mouse PRL family (Prl3c1, Prl5a1, Prl7a1, Prl7a2, Prl8a1). Furthermore, 10 of the 390 DEGs encode genes involved in placental development (Ada, Ascl2, Cdx2, Hey1, Il11ra1, Ncoa1, Pcdh12, Plac1, Syde1, Vash2).
For the male placentae, the DEGs were enriched in molecular function (structural molecule activity). Of note, the DEGs encoded transcription factors (Gata1, Pax2, Sox7, Sox11) and enzymes (Ace, Arg1, Mmp12, Nos1). Only one of the DEGs encoded a gene established in placental development (Tmed2).
Discussion
An interesting finding of the present study is that uterine glands secrete or produce factors under the control of FOXA2 that impact uterine decidualization, placental growth and differentiation, and embryo growth. In the mouse, uterine glands are predominantly present in the AM endometrium of uterus and remain active during pregnancy (18, 19). With the exception of LIF, the biological roles of other GE-derived factors and products have not been established. Recent results support the idea that uterine glands vectorially secrete many factors in a basolateral manner that govern stromal cell decidualization (49). In glandless PgrCre/+Foxa2f/f mice, intraperitoneal injections of LIF induce embryo implantation (17, 18). Although a PTGS2-expressing PDZ formed adjacent to the implanting embryos, the SDZ of LIF-replaced glandless PgrCre/+Foxa2f/f mice on GD 5.5 were abnormal based on decreased decidual marker gene expression (Alpl, Bmp2, Bmp8a, Fstl1, Prl8a2, Wnt4). BMP2 and WNT4 are key regulators of stromal cell decidualization (50, 51). In our previous study, complete embryo resorption occurred by GD 9.5 in LIF-replaced glandless PgrCreFoxa2f/f mice due to defects in the AM decidua (17). In contrast, our previous studies (17, 18) and the present study found that decidualization progresses in the AM and M regions of the uterus in LIF-replaced adult FOXA2 cKO (LtfiCre/+Foxa2f/f) mice that have glands. On GD 5.5, some decidual marker genes (Bmp2, Fstl1, Prl8a2, Wnt4) were reduced in adult FOXA2 cKO as compared to control mice. By GD 9.5, the implantation sites of LIF-replaced adult FOXA2 cKO mice were histologically normal, although a few of the decidual marker genes (Alpl, Bmp2, Bmp8a, Prl8a2, Wnt4) were decreased based on quantitative PCR results. In the present study on GD 8.5, Prl8a2 and Ptgs2 were decreased, but not absent, in the AM decidua, and Wnt4 was increased in the M decidua of LIF-replaced adult FOXA2 cKO mice. PTGS2 is a rate-limiting enzyme up-regulated in decidualizing stromal cells and required for implantation and placentation in mice (52, 53). Collective results from studies support the idea that uterine gland secretions and products are requisite for stromal cell decidualization and pregnancy establishment. Although FOXA2-dependent uterine gland secretions and products influence stromal cell decidualization, they are optional for pregnancy establishment in mice.
Similar to a recent RNA-seq study (54), substantial gene-expression differences were identified between the AM and M decidua in both control and FOXA2 cKO mice. Within both the AM and M decidua, increases and decreases in gene expression were observed between control and FOXA2 cKO mice. Functional enrichment analysis of those DEGs indicates that the AM and M decidua are distinctively altered by the lack of FOXA2-dependent secretions and products of the uterine glands. Consistently, alterations in PRL signaling were uncovered in the GD 8.5 AM and M decidua with members of the expanded PRL family increased in FOXA2 cKO mice. The expanded mouse PRL gene family encodes hormones/cytokines associated with pregnancy that are produced by the uterine decidua and trophoblast cells (55, 56) and have biological roles in blood vessel and hematopoietic cell development (57, 58). Of note, the biological activities of some expanded PRL family paralogs are important for uteroplacental adaptations to physiological stressors (55, 59, 60). For example, PRL8A2 is a secreted heparin-binding cytokine involved in pregnancy-dependent adaptations to hypoxia by restraining activation of decidual endoplasmic reticulum stress (60, 61). The increase in expression of many members of the expanded PRL family in the AM and M decidua, as well as other genes (Ptgs2, Sfrp5, Wnt4), as an adaptive response to the lack of FOXA2-dependent secretions and products of the uterine glands, may be required to maintain homeostasis of decidual function for placental development and pregnancy progression in LIF-replaced FOXA2 cKO mice.
Our previous studies found that expression of several trophoblast marker genes (Ascl2, Cited2, Ctsq, Esrrb, Hand1, Imfa, Tpbpa) were reduced in the GD 9.5 placenta of FOXA2 cKO mice (18) and over 1,000 genes were different in the placenta between control and FOXA2 cKO dams on GD 15 (38). An unexpected finding of the present study was sexual dimorphism in the GD 12.5 and GD 17.5 placenta and fetus in response to the loss of FOXA2 in uterine glands of the mother. The perturbations in decidual and placental gene expression were not sufficient to compromise pregnancy outcome and fetal growth, but they did modify fetoplacental growth in a sex-dependent manner. On GD 12.5, both the male and female fetus weighed less in LIF-replaced FOXA2 cKO dams. In contrast, the weight of the male, but not female fetus, was reduced on GD 17.5. Furthermore, female placenta weight was increased on GD 12.5 in FOXA2 cKO dams, and both female and male placentae were heavier on GD 17.5. Thus, the ratio of fetus to placenta weight, an indicator of placental efficiency, was reduced in males on GD 12.5 and both females and males on GD 17.5. Consistently, the placental transcriptome was more altered by the loss of uterine gland FOXA2 in the female as compared to male placenta on GDs 12.5 and 17.5.
Substantial alterations in the expanded PRL family were identified in the GD 12.5 and GD 17.5 placenta transcriptome of FOXA2 cKO dams. On GD 12.5, expression of PRL family members were altered in the female, but not male, placenta; however, PRL family members were altered in both female and male placentae on GD 17.5. A number of different PRL family members are expressed in endovascular and interstitial invasive trophoblast cells of the developing and functional mouse placenta (55, 62). Most members of the expanded PRL family do not activate their targets through the PRL receptor and instead possess nonclassical modes of action (63). The nonclassical PRL family members possess a broad range of pregnancy-associated cellular targets and actions, influencing vascular remodeling, hematopoiesis, immune cell function, and adaptations to physiological stressors (55, 62). For example, mice lacking Prl7b1 exhibit defects in placental adaptations to physiological stressors, such as hypoxia (64). The increased expression of many expanded PRL family members in the female and male placenta, as well as other genes, such as the solute carrier (SLC) group of membrane transport proteins, as adaptations to the lack of FOXA2-dependent secretions and products of the uterine glands may be required to maintain placental development and function for pregnancy maintenance to term in FOXA2 cKO mice. However, the compensatory mechanisms operating in the female as compared to the male placenta of FOXA2 cKO dams are different.
In other tissues and organs, FOXA factors and their targets display sexual dimorphism (41, 42), and sex-specific differences exist in fetoplacental development and response of the placenta and fetus to maternal and environmental stressors (9, 43, 44). The considerably larger change in the transcriptome of the female as compared to male placenta (628 vs. 19 DEGs) in the present study signifies that the female placenta exhibits greater plasticity and adaptability to the loss of FOXA2-dependent secretions and products of the uterine glands. Indeed, growth of the male, but not female fetus, remained diminished on GD 17.5 in FOXA2 cKO dams, indicating the female fetus is capable of greater compensatory growth during later pregnancy, likely due to suitable placental adaptations. The placenta contains distinct layers of differentiated trophoblast cells, each having specialized functions to support a pregnancy (8), including trophoblast giant cells, spongiotrophoblasts, glycogen trophoblasts, and syncytiotrophoblasts with each characterized by its unique gene expression signatures. The placenta labyrinth zone represents the principal site of hematotrophic exchange (65) between the mother and fetus, and the JZ has progenitor cells and represents the endocrine compartment (9). The relative area of the JZ compartment was increased in the female placenta of FOXA2 cKO dams at both time points of gestation and corresponded with an increase in PRL family member cytokines Prl3d1 (placental lactogen-I; giant cells) and Prl6a1 (glycogen cells), but a decrease in Prl8a6, Prl8a1, and Prl8a8 that are expressed by spongiotrophoblast cells in the JZ at GD 12.5. None of those Prl genes were altered in male placenta at this time point, further highlighting the innate adaptive capacity of the female placenta (55). Although differences in overall placenta histoarchitecture were observed, transcriptome analysis found that few specialized trophoblast cells were altered based on marker gene analysis. For example, Prld3d1 and Nr6a1 (syncytiotrophoblast) were increased in GD 12.5 female placentae of FOXA2 cKO dams, and Cdx2, Prl3c1 (spiral artery, parietal, and canal trophoblast giant cell subtypes), and Pcdh12 (glycogen cells) increased and Pecam1 (endothelial cells) decreased in GD 17.5 female placentae of FOXA2 cKO dams. However, the bulk of trophoblast marker genes were not altered in either the female or male placentae of FOXA2 cKO dams. Despite the early adaptive changes observed in the GD 12.5 female placenta, both the male and female fetuses from FOXA2 cKO dams were smaller as compared to the controls. Compensatory growth of the female but not male fetus was observed by GD 17.5 in FOXA2 cKO dams, which was correlated with the JZ expansion capability as well as substantial alterations in the placenta transcriptome. These findings support the idea that the programming effect of maternal uterine FOXA2 ablation initiates earlier than GD 12.5, apparently during development of the DB. Improper development of the decidua can lead to recurrent pregnancy loss (14), as well as later pregnancy complications including preeclampsia in humans (11, 13).
An important yet unanswered question is whether the male and female offspring of FOXA2 cKO dams will exhibit aspects of fetal programming due to intrauterine growth restriction, which is a primary risk factor for the development of adult disease and reductions in lifespan (43, 66). Sexual dimorphism in the formation and function of the placenta and fetus occurs in laboratory animals and humans (43, 44). Furthermore, sexually dimorphic effects on the placenta and fetus can result from a variety of maternal stressors, such as poor maternal diet, exposure to glucocorticoids, and hypoxia, and maternal age is observed in laboratory animals, nonhuman primates, and humans (66). Despite similar exposures, males and females often have altered disease susceptibility or progression from different life stages. This can be traced back to the in utero environment, where male fetuses are more susceptible to developmental perturbations than females (66). Future studies will need to determine the effects of uterine FOXA2 ablation and, by inference, FOXA2-dependent GE secretions and products on decidualization and placental function, as well as neonatal and adult outcomes. These findings support the idea that uterine gland dysfunction may be a critical component in the negative impacts of different effectors (maternal age, subfertility, hormonal imbalance, developmental defects, FOXA2 mutations), on fetoplacental growth and development that has relevance to developmental origins of health and disease concept.
Materials and Methods
Animals.
Animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Missouri and were conducted according to the NIH Guide for the Care and Use of Laboratory Animals (67). Floxed Foxa2f/f mice (stock no. 022620) and LtfiCre mice (stock no. 026030) were obtained from The Jackson Laboratory. Floxed Foxa2 mice (68) were crossed with or LtfiCre (37) mice to generate conditional knockout animals. Gestational time points were obtained by the mating of 8- to 10-wk-old control (Foxa2f/f) and FOXA2 cKO (LtfiCr/+Foxa2f/f) females to wild-type CD-1 male mice with the day that a vaginal plug was observed was considered day 0.5 of gestation. For FOXA2 cKO dams, mice received intraperitoneal injections of recombinant mouse LIF (10 μg in saline; catalog #554008, BioLegend) at 1000 hours and 1800 hours on GD 3.5 (17, 18).
Evaluation of the Uterus on GD 8.5.
Full details of evaluation of fetal and placental development on GDs 12.5 and 17.5, immunofluorescence analyses, morphometric measurements, transcriptome analysis, and statistical analyses can be found in SI Appendix, Supporting Materials and Methods.
Supplementary Material
Acknowledgments
We thank members of the T.E.S. laboratory for helpful discussions. This work was supported by NIH Grant R01 HD096266 from the Eunice Kennedy Shriver National Institute of Child Health and Development. A.M.K. is supported by National Research Service Award F32 HD100103 from the Eunice Kennedy Shriver National Institute of Child Health and Development.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014272117/-/DCSupplemental.
Data Availability.
Sequencing data have been deposited in the Gene Expression Omnibus (GEO), accession no. GSE154026 and in Dryad (https://datadryad.org/stash/dataset/doi:10.5061/dryad.bvq83bk69).
References
- 1.Cha J., Sun X., Dey S. K., Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 18, 1754–1767 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Dey S. K., Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 7, 185–199 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Ye X., Uterine luminal epithelium as the transient gateway for embryo implantation. Trends Endocrinol. Metab. 31, 165–180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S. K., Regional development of uterine decidualization: Molecular signaling by Hoxa-10. Mol. Reprod. Dev. 77, 387–396 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paria B. C., Reese J., Das S. K., Dey S. K., Deciphering the cross-talk of implantation: Advances and challenges. Science 296, 2185–2188 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsohn P. A., Zorn T. M., Implantation and decidualization in rodents. J. Exp. Zool. 266, 603–628 (1993). [DOI] [PubMed] [Google Scholar]
- 7.Lima P. D., Zhang J., Dunk C., Lye S. J., Croy B. A., Leukocyte driven-decidual angiogenesis in early pregnancy. Cell. Mol. Immunol. 11, 522–537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemberger M., Hanna C. W., Dean W., Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 21, 27–43 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Woods L., Perez-Garcia V., Hemberger M., Regulation of placental development and its impact on fetal growth-new insights from mouse models. Front. Endocrinol. (Lausanne) 9, 570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woods L. et al., Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat. Commun. 8, 352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrido-Gomez T. et al., Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl. Acad. Sci. U.S.A. 114, E8468–E8477 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebastian-Leon P., Garrido N., Remohí J., Pellicer A., Diaz-Gimeno P., Asynchronous and pathological windows of implantation: Two causes of recurrent implantation failure. Hum. Reprod. 33, 626–635 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Conrad K. P., Evidence for corpus luteal and endometrial origins of adverse pregnancy outcomes in women conceiving with or without assisted reproduction. Obstet. Gynecol. Clin. North Am. 47, 163–181 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas E. S. et al., Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 3, 37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelleher A. M., DeMayo F. J., Spencer T. E., Uterine glands: Developmental biology and functional roles in pregnancy. Endocr. Rev. 40, 1424–1445 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelleher A. M. et al., Integrative analysis of the forkhead box A2 (FOXA2) cistrome for the human endometrium. FASEB J. 33, 8543–8554 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelleher A. M., Milano-Foster J., Behura S. K., Spencer T. E., Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success. Nat. Commun. 9, 2435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelleher A. M. et al., Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc. Natl. Acad. Sci. U.S.A. 114, E1018–E1026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan J. et al., Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat. Commun. 9, 603 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora R. et al., Insights from imaging the implanting embryo and the uterine environment in three dimensions. Development 143, 4749–4754 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton G. J., Watson A. L., Hempstock J., Skepper J. N., Jauniaux E., Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 87, 2954–2959 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Gray C. A., Burghardt R. C., Johnson G. A., Bazer F. W., Spencer T. E., Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124, 289–300 (2002). [PubMed] [Google Scholar]
- 23.Gray C. A. et al., Endometrial glands are required for preimplantation conceptus elongation and survival. Biol. Reprod. 64, 1608–1613 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Filant J., Spencer T. E., Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus. Biol. Reprod. 88, 93 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Jeong J. W. et al., Foxa2 is essential for mouse endometrial gland development and fertility. Biol. Reprod. 83, 396–403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooke P. S. et al., Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol. Reprod. 86, 63 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart C. L., The role of leukemia inhibitory factor (LIF) and other cytokines in regulating implantation in mammals. Ann. N. Y. Acad. Sci. 734, 157–165 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Stewart C. L. et al., Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Friedman J. R., Kaestner K. H., The Foxa family of transcription factors in development and metabolism. Cell. Mol. Life Sci. 63, 2317–2328 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaestner K. H., The FoxA factors in organogenesis and differentiation. Curr. Opin. Genet. Dev. 20, 527–532 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filant J., Lydon J. P., Spencer T. E., Integrated chromatin immunoprecipitation sequencing and microarray analysis identifies FOXA2 target genes in the glands of the mouse uterus. FASEB J. 28, 230–243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villacorte M. et al., β-Catenin signaling regulates Foxa2 expression during endometrial hyperplasia formation. Oncogene 32, 3477–3482 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Soyal S. M. et al., Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41, 58–66 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Chen J. R. et al., Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 141, 4365–4372 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Bhatt H., Brunet L. J., Stewart C. L., Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc. Natl. Acad. Sci. U.S.A. 88, 11408–11412 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fouladi-Nashta A. A. et al., Characterization of the uterine phenotype during the peri-implantation period for LIF-null, MF1 strain mice. Dev. Biol. 281, 1–21 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Daikoku T. et al., Lactoferrin-iCre: A new mouse line to study uterine epithelial gene function. Endocrinology 155, 2718–2724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behura S. K., Kelleher A. M., Spencer T. E., Evidence for functional interactions between the placenta and brain in pregnant mice. FASEB J. 33, 4261–4272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filant J., Spencer T. E., Cell-specific transcriptional profiling reveals candidate mechanisms regulating development and function of uterine epithelia in mice. Biol. Reprod. 89, 86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelleher A. M., Burns G. W., Behura S., Wu G., Spencer T. E., Uterine glands impact uterine receptivity, luminal fluid homeostasis and blastocyst implantation. Sci. Rep. 6, 38078 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z., Tuteja G., Schug J., Kaestner K. H., Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell 148, 72–83 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugathan A., Waxman D. J., Genome-wide analysis of chromatin states reveals distinct mechanisms of sex-dependent gene regulation in male and female mouse liver. Mol. Cell. Biol. 33, 3594–3610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenfeld C. S., Sex-specific placental responses in fetal development. Endocrinology 156, 3422–3434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clifton V. L., Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 31, S33–S39 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Ge S. X., Jung D., Yao R., ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 36, 2628–2629 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erlebacher A., Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 31, 387–411 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Bulmer D., Peel S., Stewart I., The metrial gland. Cell Differ. 20, 77–86 (1987). [DOI] [PubMed] [Google Scholar]
- 48.Picut C. A., Swanson C. L., Parker R. F., Scully K. L., Parker G. A., The metrial gland in the rat and its similarities to granular cell tumors. Toxicol. Pathol. 37, 474–480 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Filant J., Spencer T. E., Uterine glands: Biological roles in conceptus implantation, uterine receptivity and decidualization. Int. J. Dev. Biol. 58, 107–116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franco H. L. et al., WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 25, 1176–1187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Q. et al., Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J. Biol. Chem. 282, 31725–31732 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Chakraborty I., Das S. K., Wang J., Dey S. K., Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J. Mol. Endocrinol. 16, 107–122 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Lim H. et al., Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91, 197–208 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Zhao M., Zhang W. Q., Liu J. L., A study on regional differences in decidualization of the mouse uterus. Reproduction 153, 645–653 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Soares M. J., Konno T., Alam S. M., The prolactin family: Effectors of pregnancy-dependent adaptations. Trends Endocrinol. Metab. 18, 114–121 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Soares M. J., Faria T. N., Roby K. F., Deb S., Pregnancy and the prolactin family of hormones: Coordination of anterior pituitary, uterine, and placental expression. Endocr. Rev. 12, 402–423 (1991). [DOI] [PubMed] [Google Scholar]
- 57.Lin J., Linzer D. I., Induction of megakaryocyte differentiation by a novel pregnancy-specific hormone. J. Biol. Chem. 274, 21485–21489 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Bittorf T. et al., Induction of erythroid proliferation and differentiation by a trophoblast-specific cytokine involves activation of the JAK/STAT pathway. J. Mol. Endocrinol. 25, 253–262 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Ain R., Dai G., Dunmore J. H., Godwin A. R., Soares M. J., A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc. Natl. Acad. Sci. U.S.A. 101, 16543–16548 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alam S. M. et al., A uterine decidual cell cytokine ensures pregnancy-dependent adaptations to a physiological stressor. Development 134, 407–415 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Alam S. M., Konno T., Soares M. J., Identification of target genes for a prolactin family paralog in mouse decidua. Reproduction 149, 625–632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai G., Lu L., Tang S., Peal M. J., Soares M. J., Prolactin family miniarray: A tool for evaluating uteroplacental-trophoblast endocrine cell phenotypes. Reproduction 124, 755–765 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Soares M. J., The prolactin and growth hormone families: Pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod. Biol. Endocrinol. 2, 51 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bu P., Alam S. M., Dhakal P., Vivian J. L., Soares M. J., A prolactin family paralog regulates placental adaptations to a physiological stressor. Biol. Reprod. 94, 107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georgiades P., Ferguson-Smith A. C., Burton G. J., Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23, 3–19 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Kalisch-Smith J. I., Simmons D. G., Dickinson H., Moritz K. M., Review: Sexual dimorphism in the formation, function and adaptation of the placenta. Placenta 54, 10–16 (2017). [DOI] [PubMed] [Google Scholar]
- 67.National Research Council , Guide for the Care and Use of Laboratory Animals, (National Academies Press, Washington, DC, 8th Ed., 2011). [Google Scholar]
- 68.Sund N. J. et al., Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyte. Mol. Cell. Biol. 20, 5175–5183 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data have been deposited in the Gene Expression Omnibus (GEO), accession no. GSE154026 and in Dryad (https://datadryad.org/stash/dataset/doi:10.5061/dryad.bvq83bk69).