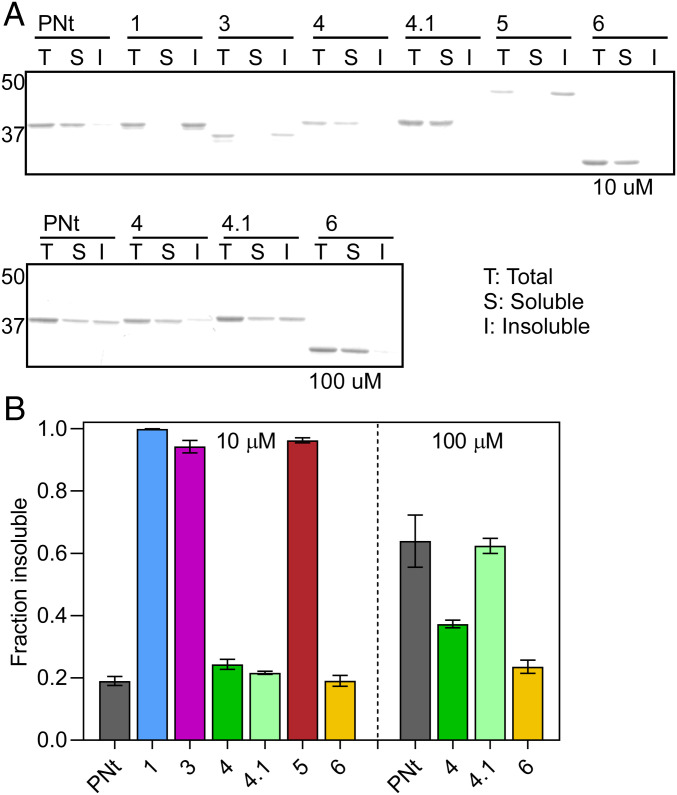
Fig. 4.
Collapsed swap variants are more prone to aggregation. (A) Proteins were incubated at 37 °C for 16 to 20 h at either 10 or 100 μM. Soluble and insoluble (aggregated) fractions were separated by centrifugation and subjected to SDS/PAGE. Gel mobility differences for some swap variants are attributed to the altered distributions of charged residues. The actual molecular weight of each construct was confirmed by intact mass spectrometry. (B) Quantification of SDS/PAGE bands from A. Each data point represents three replicates; error bars represent the SEM.