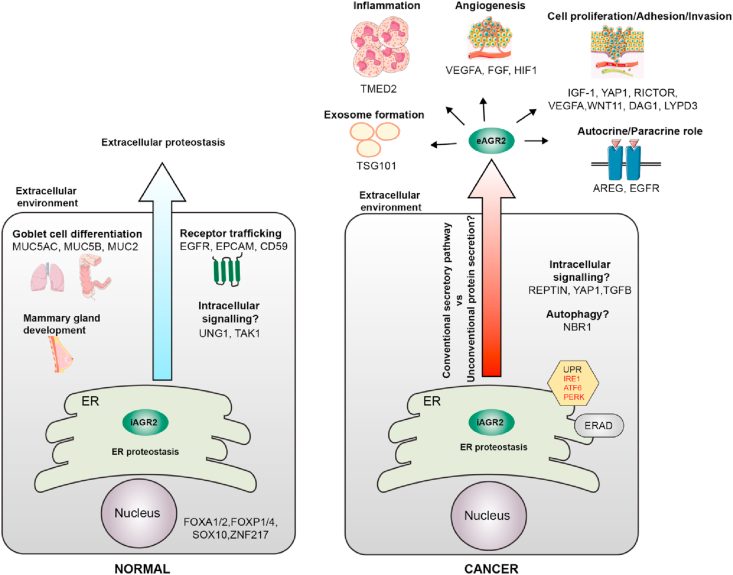
Figure 4.
Emerging roles of iAGR2 and eAGR2 in normal and cancer conditions. AGR2 normally resides in the ER predominantly in its dimeric form and involves in the ER proteostasis through its protein disulphide isomerase activity. In a normal condition, AGR2 engages in the goblet differentiation that is responsible for secreting mucus in the respiratory and intestinal tracts that protects them from pathogenic infection. AGR2 is also essential for milk production during normal mammary gland development as well as receptor maturation and trafficking. In cancer cells, ER protein synthesis machinery is challenged with high mutant protein folding demands causing an ER stress that in turns activates UPR. In this context, AGR2 is actively regulated by the UPR pathways possibly via the IRE1α and ATF6 arms that can impact on AGR2 functions and export from the ER. There could also be an interplay of UPR/ERAD/Autophagy pathways. AGR2 can also escape the ER and be secreted into the extracellular environment in cancer. The presence of eAGR2 in the extracellular environment can contribute to the hallmarks of cancers such as enhancing cell proliferation, metastasis and dissemination, inflammation and angiogenesis. The mechanisms of AGR2 secretion are beginning to be elucidated. These include, among others, the association of its structure-function variants, possible PTMs and dimeric-monomeric regulations.