Abstract
Background:
Functional and developmental versatility of mesenchymal stem cells (MSCs) have generated great interest in their clinical application. Recently, it has been proposed that the non-adherent population of bone marrow cells can differentiate to MSCs in vitro. The present study aimed to compare the anti-inflammatory potentials of adherent and non-adherent MSCs in an experimental model of ulcerative colitis (UC) in rats.
Methods:
The present experimental study was conducted at the School of Veterinary Medicine, Urmia University (Urmia, Iran) during March-May 2018. UC was induced using acetic acid in three groups of male Wistar rats, namely the control colitis, adherent MSCs treated, and non-adherent MSCs treated groups. Adherent and non-adherent MSCs were collected, characterized, and proliferated. The isolated cells were injected into the peritoneum of the respective groups of colitis rats. After 10 days, the animals were evaluated for gross and microscopic pathology, production of inflammatory mediators, and stress oxidative profile in the gut tissue. The statistical analysis was performed using SPSS software (version 23.0). P<0.05 was considered statistically significant.
Results:
The non-adherent MSCs had almost similar therapeutic potency compared to the adherent MSCs (P=0.12). They significantly reduced the level of inflammatory mediators and improved the oxidative stress profile in colonic tissue compared to the control colitis group (P=0.0001).
Conclusion:
The molecular assays and histopathological assessment revealed that the non-adherent MSCs not only had anti-inflammatory and regulatory potency but also enhanced tissue regeneration in UC rats. Therefore, the non-adherent fraction of bone marrow-derived MSCs could be used as a complementary source of MSCs in stem cell therapies.
Keywords: Mesenchymal stem cells , Colitis , Inflammation , Oxidative stress
What’s Known
Bone marrow-derived mesenchymal stem cells (MSCs) are an ideal candidate for cell therapy in ulcerative colitis.
MSC therapy enhances microcirculation and tissue regeneration in a murine model of inflammatory bowel disease.
What’s New
Non-adherent bone marrow cell-derived MSCs regulate local inflammation and improve tissue regeneration in an animal model of ulcerative colitis.
Non-adherent MSCs could be considered as a complementary source of MSCs in clinical applications.
Introduction
Bone marrow-derived mesenchymal stem cells (MSCs) are considered a promising cell source for the treatment of a variety of diseases associated with inflammation, tissue damage, and autoimmune diseases. The current enthusiasm around MSCs is due to some superior features of these cells, including anti-inflammatory and immunoregulative capacities, ease of isolation, and rapid growth. 1
Nearly half a century ago, Friedenstein and others described MSCs as precursor cells for multiple mesenchymal cell lineages in the bone marrow. In the following years, these cells were mainly characterized based on their properties in vitro and in vivo. 2 The term colony-forming unit fibroblasts (CFU-Fs) was first used by Friedenstein to specify the isolated bone marrow cells, which were adherent, fibroblastic, and clonogenic in nature. 3 In the ensuing decades, various studies precisely characterized CFU-Fs and demonstrated that under specific conditions a proportion of these cells could give rise to multiple mesenchymal tissues. 4 Hereupon, the generic term MSC was used to refer to all bone marrow CFU-Fs. Several studies documented that adult bone marrow-derived MSCs could also differentiate into the cells of other developmental lineages, such as visceral mesoderm, neuroectoderm, and endoderm. 5
According to Friedenstein and others, MSCs are highly adherent fibroblast-like cells that attach to plastic plates within 24 hours after primary culture. 6 This made scientists to exclusively consider MSCs as a highly adherent fibroblast-like cell population and thus, limiting subsequent studies into possible alternative phenotypes of MSCs in the bone marrow. Recently, several studies have indicated that the non-adherent population of bone marrow-derived mesenchymal cells (referred to as non-adherent MSCs) might be a complementary source for MSCs. This fraction can give rise to multiple mesenchymal lineages in vitro and form distinct mesenchymal tissue, such as skeletal muscle and bone in vivo after transplantation. 7 - 9 Although bone marrow is considered the most abundant source of MSCs, the absolute number of MSCs remains low per aspiration, which is a limiting factor in its clinical application. Furthermore, even though the culture of adequate quantities of adherent MSCs for immunotherapy is time-consuming, a lion’s share of non-adherent MSCs or their precursors is discarded as waste during the medium exchange. 10 - 12
Ulcerative colitis (UC) is an idiopathic form of inflammatory bowel disease that affects the colonic and ileal mucosa. 13 Although the principal etiology of the disease is unknown, the spate of ailments predominantly relies on a disrupted response of the immune system to gut microbiome, due to enhanced inflammatory signaling pathways, leading to continuous inflammation. 14 As a result of advancements in stem cell-based therapy, bone marrow MSCs have been considered a potential candidate for cell therapy in chronic inflammatory diseases such as UC. 15 , 16 It has been reported that adherent MSCs can improve the clinical manifestation of UC. Yet, it is unclear whether non-adherent MSCs possess the same potential. Based on the findings of previous studies, we hypothesized that non-adherent MSCs can modulate inflammation just as their adherent peers can. To this end, we developed an animal model of UC to determine whether non-adherent bone marrow-derived MSCs can equally quench the flame of inflammation.
Materials and Methods
Reagents
The total protein assay kit was purchased from Zist Chemi Co. (Tehran, Iran). Fetal calf serum and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from GIBCO, Life Technologies Inc. (Gaithersburg, Maryland, USA). The enzyme-linked immunosorbent assay (ELISA) kits were purchased from Peprotech (UK). Other reagents were acquired from Sigma-Aldrich (St. Louis, Missouri, USA).
Animals
A total of 50 male Wistar albino rats, aged 6 weeks and weighing 200-250 g, were obtained from the experimental animal care center of Urmia University, Urmia, Iran. The animals were housed under controlled environmental conditions (25 °C and 12-hour light: dark cycle) in plastic boxes lined with wood shavings and received food and water ad libitum. All procedures were carried out according to the animal care code of practice of Veterinary Medicine School, Urmia University, Urmia, Iran, (ethical code: IR-UU-AEC-1116/AD3). Ten rats were sacrificed to collect bone marrow MSCs and 30 rats were prepared as models of UC for in vivo experiments. The remaining 10 rats were used as the control group.
Colitis Induction and Experimental Groups
Acute colitis was induced as described in a previous study. 17 In brief, all rats fasted for 36 hours before the induction of colitis. Under light ether anesthesia, a pediatric catheter was inserted into the colon such that its tip was 8 cm proximal to the anus. Thereafter, 4 ml diluted acetic acid (4%) solution was administered by an intracolonic enema. The control rats were also subjected to intracolonic administration of 4 ml normal saline 0.9%. Finally, the animals were held in a vertical head-down position for 1 minute to prevent acetic acid leakage.
Thirty colitis rats were randomly assigned into three groups (10 rats per group), namely colitis control, adherent MSCs treated, and non-adherent MSCs treated groups. The colitis control group intraperitoneally received only phosphate-buffered saline (PBS). Both the adherent and non-adherent MSCs treated groups intraperitoneally received 2×106 cells. The animals were housed in plastic cages with a maximum of 5 rats per cage. On day 10 after MSC transplantation, the animals were sacrificed and gut tissues were harvested for further analysis.
Isolation and Proliferation of Adherent and Non-Adherent MSCs
Bone marrow-derived MSCs were isolated as described in a previous study. 18 In brief, under deep anesthesia, the bone marrow of the rats’ tibias and femurs was flushed out. The obtained marrow was rinsed twice by centrifugation at 250 g for 5 minutes in PBS. The cells were plated in 75 cm2 tissue culture flasks at concentrations of 0.3×106 to 0.4×106 cells/cm2 in DMEM media, added with 15% fetal calf serum. The isolated cells were then incubated at 37 °C with 5% CO2 in a humidified incubator. Four days ensuing the culture initiation, the cell culture mediums were collected, centrifuged, and then seeded in a 75 cm2 flask. Upon 70% confluence, cells were trypsinized using trypsin-ethylenediaminetetraacetic acid (EDTA), counted, and passed in a 1:3 ratio (about 1.5×106 cells per 75 cm2 flask). The culture media were replenished every 3 days for 21 days. The cell suspension of the third generational passage was collected for administration.
To obtain non-adherent MSCs, the suspended cells in the primary culture were exploited. Four days after the primary culture, the supernatant of non-adherent cells was removed, rinsed, centrifuged at 250 ×g for 10 minutes, and transferred to another 75 cm2 flask. Moreover, non-adherent cells of the second and third media exchange were also removed and seeded in two distinct 25 cm2 flasks. 19
Characterization of MSCs
In accordance with a previous study, 1 the isolated MSCs in the third subculture and non-adherent MSCs were used for morphological identification by flow cytometry. In brief, the fluorescent-conjugated monoclonal antibodies (PE-labeled anti-CD29, FITC-labeled anti-CD45, and PCY5-labeled anti-CD90) were exploited for MSC markers staining. The cell fluorescence was immediately measured by a DAKO flow cytometer (Partec, Germany). The cells (5×105in 100 μL PBS, 0.5% bovine serum albumin (BSA), and 2 mmol EDTA) were blended with 10 μL of the fluorescently labeled monoclonal antibody (anti-rat CD29 [Integrin b chain, Ha2/5, FITC], CD45-FITC, and CD90-PCY5 [Thy-1/Thy-1.1-FITC]) and incubated in the dark for 30 minutes at 4 °C. The stained cells were rinsed twice with PBS (containing 2% BSA) and the pellet was re-suspended in PBS. Immediately, the cell fluorescence was analyzed using a DAKO flow cytometer.
To ensure the obtained cells were MSCs, their immunosuppressive effect on polyclonal T-cell proliferation in vitro after isolation was exploited. 20 In brief, splenocytes were aseptically removed from three rats. Then, 1×106 cells/mL were stimulated with phytohemagglutinin (PHA) at a final concentration of 5 µg/ml or culture medium alone and incubated in the presence (1×105cells/ml) or absence of MSCs for 5 days. Then, the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) solution (20 μL, final concentration: 5 mg/mL) was added to the culture. Four hours later, 150 ml Dimethyl sulfoxide (DMSO) was added and the solution was shaken vigorously. Finally, the optical density (OD) at 492 nm was noted using a microplate reader (Dynatech, Denkendorf, Germany). The experiments were performed in triplicate. The results were reported as the proliferation index (PI) of the MTT assay and calculated according to the ratio of OD550 of pulsed cells with PHA to non-pulsed splenocytes.
Macroscopic and Microscopic Disease Severity Score
Rectal bleeding, stool blood, and stool consistency were monitored daily. The disease severity score was estimated as the sum of the scores of the parameters (table 1). Colon tissues were removed and fixed in a 10% neutral buffered formaldehyde solution for 24 hours. Each sample was embedded in a paraffin block and stained with hematoxylin and eosin for light microscopy. Afterward, four samples were examined by a pathologist blinded to the study. The scoring system was based on the following criteria: intact epithelium, no leucocyte or hemorrhage (score 0); <25% disrupted epithelium, focal leucocyte infiltrates, and focal hemorrhage (score 1); 25% disrupted epithelium, focal leucocyte infiltrates, and focal hemorrhage (score 2); 50% disrupted epithelium, widespread leucocytes, and hemorrhage (score 3); and >50% disrupted epithelium, extensive leucocyte infiltration, and hemorrhage (score 4). 21 The histological sections were assessed by two researchers blinded to the treatment groups.
Table 1.
Disease severity scoring system
| Score | Rectal bleeding | Stool consistency | Blood |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | Red | Soft | Red |
| 2 | Dark red | Very soft | Dark red |
| 3 | Gross bleeding | Diarrhea | Black |
Preparation of Colonic Homogenate
Approximately 10 cm of the distal colonic tissue was cut, opened, and washed with PBS. The same amount of the tissue was homogenized in 10 volumes of ice-cold physiological saline and then centrifuged at 10,000 ×g at 4 °C for 10 minutes. 22
Assessment of Myeloperoxidase Activity in the Colonic Homogenate
Myeloperoxidase (MPO) has mainly been used as a biochemical indicator of granulocyte, particularly neutrophil infiltration into gastrointestinal tissues. The MPO activity level was examined in accordance with a method described earlier. In brief, 10 µL of the homogenized sample was mixed with 80 µL of 0.75 mM H2O2 and 110 µL tetramethylbenzidine (TMB) solution (2.9 mM TMB in 14.5% DMSO plus 150 mM sodium phosphate buffer at pH 5.4). The absorbance was immediately assessed at 450 nm (reference: 620 nm) on a microplate reader. The samples were then incubated at 37 °C for 15 minutes. In order to cease the reaction, 50 µL of 2M H2SO4 was added and the absorbance was read spectrophotometrically at 450 nm. For the assay, 10 µL of horseradish peroxidase (HRP), 2.5 and 25 mU/ml HRP were used. Finally, MPO activity was computed as the difference of absorbance relating to the standard curve of HRP. Data were presented as milliunits per milliliter (mU/mL). 23
Determination of Nitric Oxide Concentration in the Colonic Homogenate
Griess reagent, a widely accepted colorimetric method for measuring nitric oxide (NO) concentration, was used to detect NO in colonic tissues. In brief, 50 µL of Griess reagent (0.1% sulfanilamide, 3% phosphoric acid, and 0.1% naphthyl ethylenediamine) were coupled with 50 µL of homogenized colonic tissue and kept in the dark at 25 °C for 10 minutes. Then, the absorbance was measured at 540 nm on a microplate reader. The nitrite level was controlled in accordance with the standard curve. 24
Malondialdehyde Determination in the Colonic Homogenate
Malondialdehyde (MDA) is one of the most prevalent byproducts of lipid peroxidation during oxidative stress. MDA assessment was performed in accordance with the procedure described in a previous study. 25 According to the protocol, 2.5 ml reaction buffer (0.37% thiobarbituric acid, 0.25 M HCl, and 15% trichloroacetic acid, 1:1:1 ratio) was added to 100 μL of colon homogenate and heated at 95 °C for an hour. After warming down, the mixture was centrifuged at 3,500 ×g for 10 minutes. Finally, the absorbance of the supernatant was counted at 540 nm on a microplate reader. Data were expressed as nM of MDA/mg protein.
Assessment of Inflammatory Cytokines in Colonic Homogenate
The levels of tumor necrosis factor-alpha (TNF-α), interleukin 1-beta (IL-1β), and interleukin-6 (IL-6) in the colon samples were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (PeproTech, UK). 26
Assessment of Total Protein Concentration in the Colonic Homogenate
Assaying the level of proteins using the dye-binding method has a high level of accuracy and sensitivity. In the present study, the protein concentration was determined using pyrogallol red-molybdate assay. After the preparation of homogenized colon tissue, the total protein concentration was measured and quantified according to the manufacturer’s instructions (Zist Chemi Co, Iran). 27 The increase in absorbance at 600 nm was directly proportional to the protein concentration in the sample.
Statistical Analysis
Statistical analysis was performed using SPSS software, version 23.0 (SPSS Inc., Chicago, IL, USA). In the case of non-parametric values (discontinuous ranks related to the severity of the disease), the Kruskal-Wallis test followed by the Mann-Whitney U test with Bonferroni were applied to compare score differences between the groups. For continuous data, after confirming their normal distribution with the Kolmogorov-Smirnov test, these parametric data were assessed using the one-way analysis of variance (ANOVA) and Dunnett’s post hoc test. Data were expressed as mean±SD. P<0.05 was considered statistically significant.
Results
Characterization of MSCs
Adherent MSCs and non-adherent bone marrow cell-derived MSCs were characterized by commonly used MSCs markers (figure 1A). Flow cytometry analysis showed that neither adherent nor non-adherent MSCs expressed CD45, whereas both CD29 and CD90 (common markers for MSCs in rats) were clearly expressed in both cells (table 2). Besides, the result of MTT assay showed that adherent and non-adherent MSCs in co-culture with activated T-cells significantly suppressed T-cell proliferation (figure 1B).
Figure 1.
Isolated mesenchymal stem cells were characterized by flow cytometry assay and their immunosuppressive features. (A) Immunophenotypic characterization of isolated mesenchymal stem cells. mesenchymal stem cells expressed CD54 and CD90 but not CD45. (B) The co-culture of mesenchymal stem cells T-cells (*P<0.01 versus T-cells without mesenchymal stem cells).
Table 2.
Immunophenotypic characterization of isolated adherent and non-adherent MSCs. The result of the t test indicated no difference between the groups
| Marker | Adherent MSCs (mean±SD) | Non-adherent MSCs (mean±SD) | P value |
|---|---|---|---|
| CD29 | 96.52±1.13 | 94.34±1.41 | 0.15 |
| CD45 | 1.13±0.14 | 1.42±0.35 | 0.27 |
| CD90 | 98.09±0.75 | 96.49±1.7 | 0.09 |
Data are expressed as mean±SD; MSCs: Mesenchymal stem cells
Macroscopic and Microscopic Assessments of Colonic Tissue
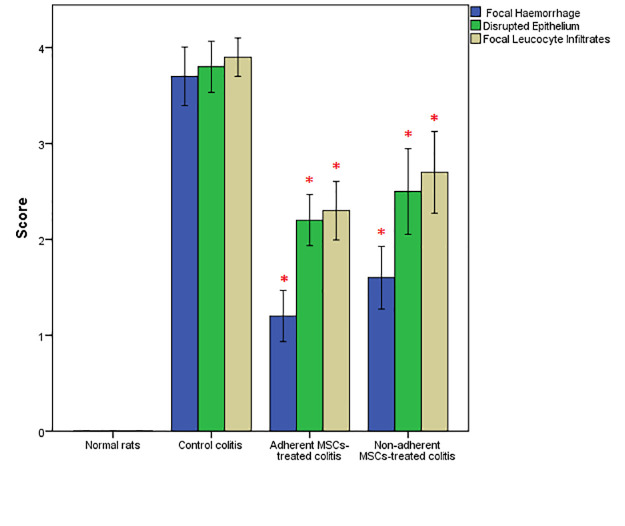
Notable manifestations of colitis were severe bloody stool and diarrhea, particularly during the first few days. However, stool consistency was improved on day 5 in both the adherent and non-adherent MSCs treated groups. Towards the end of the experiment, no gross bleeding or bloody stool was observed in either group. As a direct result, the severity disease score was improved in both the adherent and non-adherent MSCs groups after 10 days (figure 2).
Figure 2.

Both the adherent and non-adherent mesenchymal stem cells ameliorated the clinical scores of ulcerative colotis in the same manner as in the control colitis group (*P<0.001 versus control positive rats).
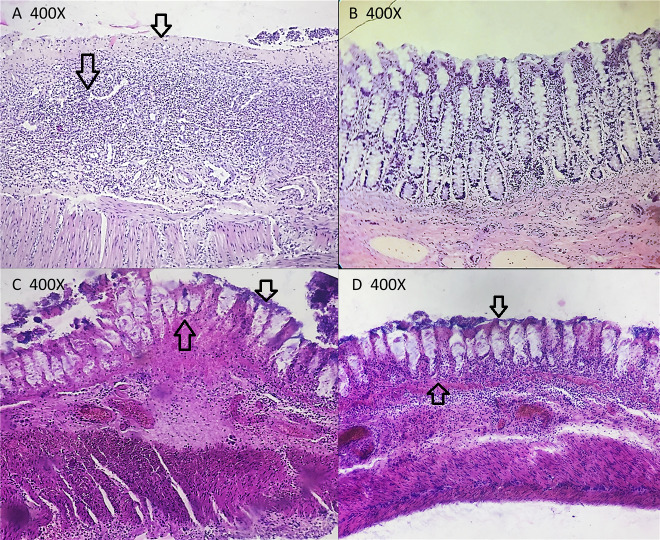
A comparison was made between the treated groups and the colitis group based on the mucosal architectural abnormalities (lamina propria cellularity, neutrophil infiltration, and epithelial abnormality). To get a better understanding of the difference between the samples, normal colon tissue was initially examined using a light microscope. Extensive mucosal damage was generally accompanied by sub-mucosal edema, hemorrhage, and inflammatory cell infiltration after acid acetic induction. As indicated by regenerated epithelium and decreased number of inflammatory cells in lamina propria, the extent of pathological changes was significantly improved in both the adherent and non-adherent MSCs groups (figures 3 and 4).
Figure 3.
Regenerated epithelium with ameliorated looking gland architecture, decreased the number of inflammatory cells in lamina propria, and decreased hemorrhage. H&E stain 400×. (C) Non-adherent mesenchymal stem cells: Significant improvement of the histological alterations is shown by partial remission. Nevertheless, the focal surface epithelial destruction can be observed. H&E stain 400×. (D) Adherent MSCs: Regenerated epithelium with ameliorated looking gland architecture, decreased the number of inflammatory cells in lamina propria, and decreased hemorrhage. H&E stain 400×.
Figure 4.
Both the adherent and non-adherent mesenchymal stem cells significantly improved histopathological scores of colitis in rats (*P<0.001 versus control positive rats).
The Condition of Inflammatory Mediators in Colonic Tissue
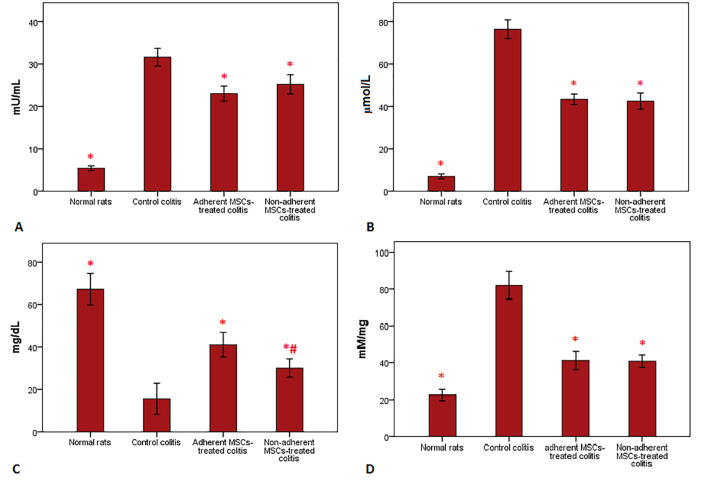
The level of MPO was significantly reduced in both the adherent and non-adherent MSCs groups compared with the control group (figure 5A). However, no significant regression was observed in the MPO activity between the treated groups. Similarly, the NO tissue concentration was significantly decreased in the treated groups compared with the control group (P<0.001); although the decrease in the adherent MSCs group was higher than in the non-adherent MSCs group (figure 5B). After cell therapy, the level of MDA in the colonic homogenate was equally reduced in both the adherent and non-adherent MSCs groups (figure 5C). In addition, the total concentration of tissue protein was significantly elevated in both treated groups compared with the control group (figure 5D).
Figure 5.
Evaluation of biochemical changes in colon tissues is shown in this figure. (A) Myeloperoxidase activity was reduced after cell injection. Both the adherent and non-adherent mesenchymal stem cells down-regulated myeloperoxidase activity in a significant manner compared with the control colitis group. (B) The levels of nitric oxide activity were down-regulated in the gut tissue of the treatment groups. There was no significant regression between the groups with adherent and non-adherent mesenchymal stem cells. (C) Administration of adherent mesenchymal stem cells and non-adherent mesenchymal stem cells markedly reduced the elevated lipid peroxidation. (D) The total protein concentration in colonic tissues differed in the treated groups. There was a significant difference between the adherent and non-adherent mesenchymal stem cells groups; nevertheless, both markedly increased the protein level compared with the control colitis group (*P<0.001 versus control positive rats).
Inflammatory Cytokines Changes in the Colonic Tissue
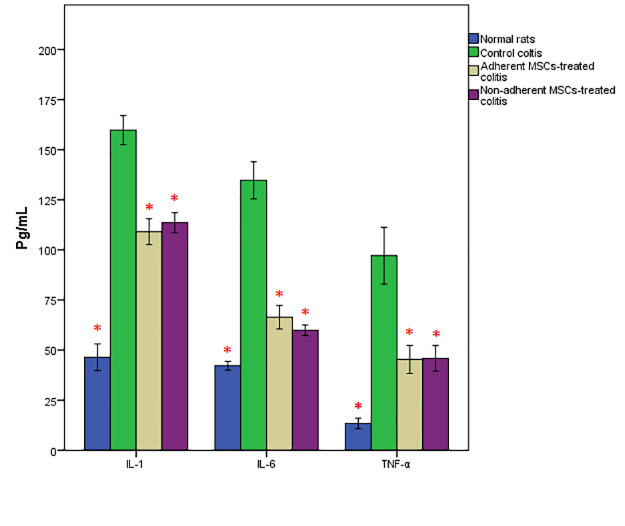
As expected, the levels of TNF-a, IL-1β, and IL-6 were noticeably elevated in the colon tissue of the colitis rats. However, after the treatment, their levels were significantly reduced in rats treated with both adherent and non-adherent MSCs. There was no significant increase in these cytokines in the specimens of the control rats (figure 6).
Figure 6.
The levels of T tumor necrosis factor-alpha, interleukin 1-beta, and interleukin-6 were significantly elevated in the colon samples of rats treated with the adherent or non-adherent mesenchymal stem cells (*P<0.001 versus control positive rats).
Discussion
UC is a chronic, disabling, and progressive disorder characterized by a lifelong treatment and has a detrimental influence on the quality of life of patients. For several decades, anti-inflammatory drugs such as 5-aminosalicylic acid and glucocorticoids have been used to treat UC. However, as a result of a better understanding of the exact pathological mechanisms of UC, different therapies such as stem cell therapy have been proposed. 28 Several studies have reported the regenerative and immunomodulatory properties of MSCs in UC models. 29 - 31 In the present study, we sought to determine whether the non-adherent fraction of MSCs could control inflammation in UC. We found that non-adherent MSCs had an almost similar therapeutic potency as the adherent MSCs.
The results showed an increase in the activity level of NO and MPO in the colonic homogenate of rats with colitis compared with the control rats. The acetic acid-induced colitis is a reliable experimental model to mimic UC pathogenesis in animal models. 32 Luminal administration of the acetic acid into the colon leads to acute local inflammation, extending into the lamina propria submucosa and external muscle layers. 33 According to the disease pathogenesis, neutrophils are the major immune cells that migrate into the colonic tissue after luminal instillation of acetic acid and cause inflammatory injuries by uncontrolled production of oxygen and nitrogen species. A previous study demonstrated that the insertion of MSCs into the inflamed tissue would reduce local inflammation by suppressing MPO and NO production and promote tissue healing by reducing lipid peroxidation. 34 Our results showed that both the adherent and non-adherent MSCs could suppress the levels of oxygen and nitrogen reactive substances in the colitis rats. Due to the involvement of free radicals in the pathogenesis of UC, it is a useful strategy for eliminating these molecules.
The hallmark of lipid peroxidation by oxygen and nitrogen species is the production of reactive aldehydes, such as malondialdehyde. 34 One of the most important findings of our study was an increase in the level of MDA in the colonic homogenates in rats with colitis and the ability of MSCs to reduce its level. In this regard, a previous study demonstrated that MSCs increased the antioxidant capacity of small bowel tissue following an intestinal ischemia-reperfusion injury. 35 We demonstrated that after the migration of MSCs into the reperfused small intestine, these cells reduced oxidative stress as a result of the effects of superoxide dismutase, catalase, and glutathione peroxidase, as well as reducing the MDA level. Moreover, the level of MDA was decreased in both the adherent and non-adherent MSCs treated groups. Therefore, we believe that some of the beneficial effects reported in the current study stem from the potent antioxidant properties of non-adherent MSCs.
It was also found that colitis reduced the total protein content of colonic homogenate. Intra-rectal administration of acetic acid solution caused a diffuse inflammation in the colon, which finally led to severe ulcerations and epithelium disruption. Free oxygen and nitrogen radicals, produced in the injured tissue, destroyed cellular integrity through nitrosation, oxidation, and chlorination of macromolecules like proteins. 36 Although we observed a significant decrease in the level of total protein in colon tissue, non-adherent MSCs increased the total protein content compared with the control colitis group. Recently, it has been reported that irradiated mice treated with MSCs had a better outcome compared with untreated mice. Additionally, the irradiated mice that received MSCs showed less intestinal injury and a significant increase in the number of regenerating crypts by amplifying activation of Wnt/β-catenin signaling in the small intestines. 37 Furthermore, reduction of the disease severity score alongside improved tissue integrity indicated that non-adherent MSCs could not only regulate inflammatory responses but also enhanced tissue healing and regeneration.
An increase in the level of inflammatory cytokines in rats with colitis was one of the expected findings of the present study. The inflammatory cytokines (IL-6, IL-1β, and TNF-α) promoted and propagated the inflammatory reaction and injurious in inflammatory bowel disease. 38 The results showed that the levels of these cytokines were down-regulated in the gut homogenate of the non-adherent MSCs treated group similar to that of the group treated with adherent MSCs. These cytokines were the key pathophysiologic elements that governed the initiation and evolution of UC. Thus, the reduction of pro-inflammatory cytokines could be a beneficial approach to control disease manifestation. It is evident that MSCs secreted anti-inflammatory molecules, which directly or indirectly suppressed the production of inflammatory cytokines. 39
Clinical application of culture-expanded bone marrow MSCs in autoimmune and autoinflammatory diseases has become widely accepted. However, obtaining a sufficient number of MSCs in a short time limits its wide clinical usage. There are different methods to expedite MSCs proliferation, such as growth factor supplements, anabolic drugs, or autologous serum. However, they might affect cell differentiation and functions after in vivo expansion and increase the risk of contamination. Therefore, isolation and culture of non-adherent MSCs allow us to increase the total number of MSCs in a short period of time.
The main limitation of the present study was the short duration (10 days) of the experimental study. It is recommended to use a UC model over a long period to achieve a higher level of data reliability.
Conclusion
The results showed that non-adherent bone marrow-derived MSCs possess favorable anti-inflammatory characteristics. They can proliferate and differentiate into the specific cells of the target tissue, and thus play a pivotal role in the tissue regeneration after administration. We also demonstrated that by collecting non-adherent MSCs, the absolute number of MSCs would increase in a short time. They may serve as a complementary source of MSCs in bone marrow, facilitating the clinical application of MSCs in the treatment of inflammatory diseases.
Acknowledgement
The present manuscript was extracted from the DVM thesis by Siavash Mashhouri. We would like to thank Urmia University for providing financial support for the study (grant number: 1529). Technical assistance by Mr. Asghar Ali-Yari is greatly appreciated.
Conflict of Interest: None declared.
References
- 1.Abbasi A, Kukia NR, Froushani SMA, Hashemi SM. Nicotine and caffeine alter the effects of the LPS- primed mesenchymal stem cells on the co-cultured neutrophils. Life Sci. 2018;199:41–7. doi: 10.1016/j.lfs.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 4.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35 doi: 10.1042/BSR20150025. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–9. doi: 10.1016/j.stem.2008.03.002. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Latzinik NV, Gorskaya Yu F, Luria EA, Moskvina IL. Bone marrow stromal colony formation requires stimulation by haemopoietic cells. Bone Miner. 1992;18:199–213. doi: 10.1016/0169-6009(92)90807-p. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Zhang J, Ben X. Neuronal-like cell differentiation of non-adherent bone marrow cell-derived mesenchymal stem cells. Neural Regen Res. 2013;8:2078–85. doi: 10.3969/j.issn.1673-5374.2013.22.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng RC, Kim SK, Heo SJ, Koak JY, Lee JH, Park JM. Characteristics and response of mouse bone marrow derived novel low adherent mesenchymal stem cells acquired by quantification of extracellular matrix. J Adv Prosthodont. 2014;6:351–60. doi: 10.4047/jap.2014.6.5.351. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolzing A, Bauer E, Scutt A. Suspension cultures of bone-marrow-derived mesenchymal stem cells: effects of donor age and glucose level. Stem Cells Dev. 2012;21:2718–23. doi: 10.1089/scd.2011.0406. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Fan Y, Wang Z, Wan Y, Zhou Z, Zhong B, et al. Isolation, characterization, and gene modification of dairy goat mesenchymal stem cells from bone marrow. In Vitro Cell Dev Biol Anim. 2012;48:418–25. doi: 10.1007/s11626-012-9530-z. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZL, Tong J, Lu RN, Scutt AM, Goltzman D, Miao DS. Therapeutic potential of non-adherent BM-derived mesenchymal stem cells in tissue regeneration. Bone Marrow Transplant. 2009;43:69–81. doi: 10.1038/bmt.2008.260. [DOI] [PubMed] [Google Scholar]
- 12.Esmaili Gourvarchin Galeh H, Meysam Abtahi Froushani S, Afzale Ahangaran N, Hadai SN. Effects of Educated Monocytes with Xenogeneic Mesenchymal Stem Cell-Derived Conditioned Medium in a Mouse Model of Chronic Asthma. Immunol Invest. 2018;47:504–20. doi: 10.1080/08820139.2018.1458108. [DOI] [PubMed] [Google Scholar]
- 13.Meier J, Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol. 2011;17:3204–12. doi: 10.3748/wjg.v17.i27.3204. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA, Jr Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2015;21:2696–707. doi: 10.1097/MIB.0000000000000543. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Zhao G, Zhang L, Qiao C, Di A, Gao H, et al. Safety and therapeutic effect of mesenchymal stem cell infusion on moderate to severe ulcerative colitis. Exp Ther Med. 2016;12:2983–9. doi: 10.3892/etm.2016.3724. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low D, Nguyen DD, Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther. 2013;7:1341–57. doi: 10.2147/DDDT.S40107. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karimi H, Emami SA, Olad-Gubad MK. Bone Marrow Stem Cells and Ear Framework Reconstruction. J Craniofac Surg. 2016;27:2192–6. doi: 10.1097/SCS.0000000000003146. [DOI] [PubMed] [Google Scholar]
- 19.Wan C, He Q, McCaigue M, Marsh D, Li G. Nonadherent cell population of human marrow culture is a complementary source of mesenchymal stem cells (MSCs) J Orthop Res. 2006;24:21–8. doi: 10.1002/jor.20023. [DOI] [PubMed] [Google Scholar]
- 20.Shushtari N, Abtahi Froushani SM. Caffeine Augments The Instruction of Anti-Inflammatory Macrophages by The Conditioned Medium of Mesenchymal Stem Cells. Cell J. 2017;19:415–24. doi: 10.22074/cellj.2017.4364. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noronha-Blob L, Lowe VC, Muhlhauser RO, Burch RM. NPC 15669, an inhibitor of neutrophil recruitment, is efficacious in acetic acid-induced colitis in rats. Gastroenterology. 1993;104:1021–9. doi: 10.1016/0016-5085(93)90269-i. [DOI] [PubMed] [Google Scholar]
- 22.Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013;19:5633–44. doi: 10.3748/wjg.v19.i34.5633. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulli B, Ali M, Forghani R, Schob S, Hsieh KL, Wojtkiewicz G, et al. Measuring myeloperoxidase activity in biological samples. PLoS One. 2013;8:e67976. doi: 10.1371/journal.pone.0067976. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–57. doi: 10.1016/j.freeradbiomed.2007.04.026. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Rejaie SS, Aleisa AM, Sayed-Ahmed MM, Al-Shabanah OA, Abuohashish HM, Ahmed MM, et al. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement Altern Med. 2013;13:136. doi: 10.1186/1472-6882-13-136. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Huang Y, Zhao Y, Hu Y, Li Z, Guo Q, et al. LL202 protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting MAPK/AP-1 signaling. Oncotarget. 2016;7:63981–94. doi: 10.18632/oncotarget.11742. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orsonneau JL, Douet P, Massoubre C, Lustenberger P, Bernard S. An improved pyrogallol red-molybdate method for determining total urinary protein. Clin Chem. 1989;35:2233–6. [PubMed] [Google Scholar]
- 28.Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA, Jr Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2015;21:2696–707. doi: 10.1097/MIB.0000000000000543. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fawzy SA, El-Din Abo-Elnou RK, Abd-El-Maksoud El-Deeb DF, Yousry Abd-Elkader MM. The possible role of mesenchymal stem cells therapy in the repair of experimentally induced colitis in male albino rats. Int J Stem Cells. 2013;6:92–103. doi: 10.15283/ijsc.2013.6.2.92. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castelo-Branco MT, Soares ID, Lopes DV, Buongusto F, Martinusso CA, do Rosario A, Jr , et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. doi: 10.1371/journal.pone.0033360. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sala E, Genua M, Petti L, Anselmo A, Arena V, Cibella J, et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology. 2015;149:163–76. doi: 10.1053/j.gastro.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Cagin YF, Parlakpinar H, Vardi N, Polat A, Atayan Y, Erdogan MA, et al. Effects of dexpanthenol on acetic acid-induced colitis in rats. Exp Ther Med. 2016;12:2958–64. doi: 10.3892/etm.2016.3728. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–88. doi: 10.4196/kjpp.2014.18.4.279. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JY, Kang HJ, Hong JS, Kim CJ, Shim JY, Lee CW, et al. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017;7:9412. doi: 10.1038/s41598-017-09827-5. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inan M, Bakar E, Cerkezkayabekir A, Sanal F, Ulucam E, Subasi C, et al. Mesenchymal stem cells increase antioxidant capacity in intestinal ischemia/reperfusion damage. J Pediatr Surg. 2017;52:1196–206. doi: 10.1016/j.jpedsurg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Tian T, Wang Z, Zhang J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid Med Cell Longev. 2017;2017:4535194. doi: 10.1155/2017/4535194. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong W, Guo M, Han Z, Wang Y, Yang P, Xu C, et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 2016;7:e2387. doi: 10.1038/cddis.2016.276. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–67. doi: 10.1053/j.gastro.2011.02.016. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552–70. doi: 10.4252/wjsc.v6.i5.552. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]