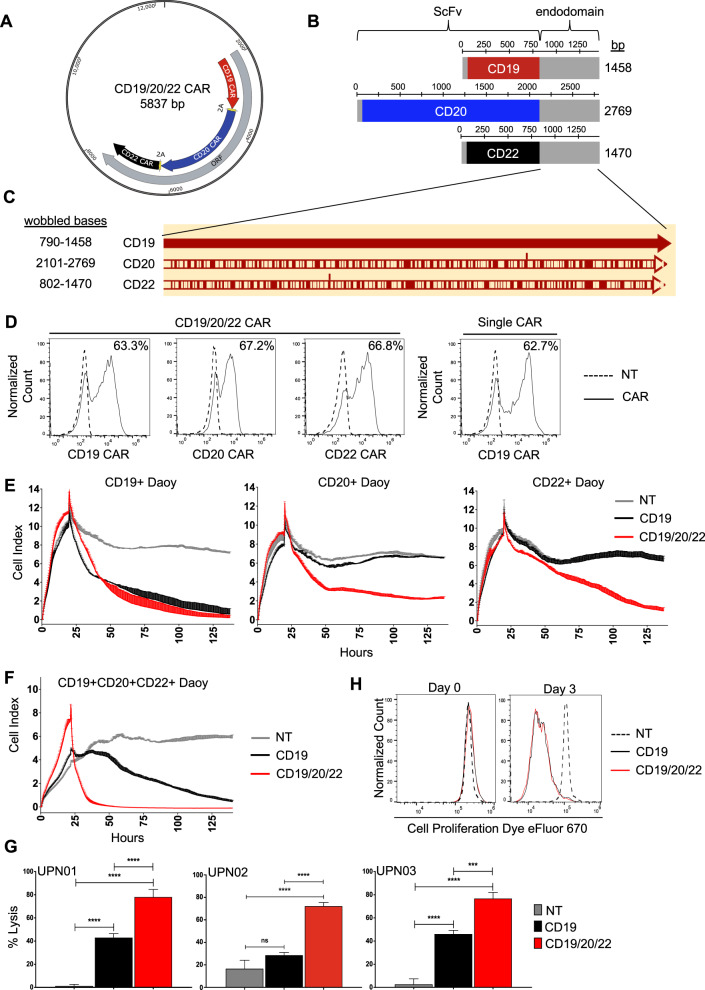
Fig. 2. Design of CD19/20/22CAR T-cells.
a A tricistronic vector was designed with self-cleaving 2A peptides, enabling trivalent protein expression of CD19/20/22-directed CARs on T-cells. b Design of CAR transgenes. Each CAR endo-domain contains a CD8α hinge and transmembrane region followed by downstream 4-1BB and CD3ζ intracellular signaling domains. c Diagram of DNA wobbling of CAR endo-domain transgenes. Common segments of DNA were wobbled so that no more than 20 consecutive base pairs are the same in any of the three transgenes. Using the CD19CAR sequence as a reference, red bars on the CD20 and CD22 CARs indicate the positions of DNA wobbling. d Flow cytometry was performed on T-cells ~1 week after retroviral CAR transduction. Results demonstrate specific binding to each individual scFv region with detection methods unique to each CAR (Fig. S1B). Histograms shown are representative data. Long-term impedance-based xCELLigence killing assay targeting Daoy tumor cells (Fig. S2A, B) expressing each target antigen singly (n = 2) (e) or all three antigens simultaneously (n = 2) (f). Tumor cells adhered and expanded for 24 h before CAR T-cells were added in a 1:3 E:T ratio. NT T-cells serve as a negative control. A decreasing cell index indicates tumor lysis. g Four hours 51Cr release assay targeting B-lineage BL-ALL cells, UPN01, UPN02, and UPN03, at an E:T ratio of 3:1 (n = 3). NT T-cells serve as a negative control. Data represent the mean of triplicate samples +SD; *p < 0.05, ***p < 0.001, ****p < 0.0001, one-way ANOVA with Tukey’s multiple comparison post-test. h NT, CD19CAR, and CD19/20/22CAR T-cells were stained with eFluor 670 proliferation dye, and proliferation capacity of CAR-expressing T-cells was assessed over 72 h of exposure to BL-ALL cells (n = 2).