Fig. 2. Endoplasmic reticulum (ER) stress-induced unfolded protein response (UPR) signalling.
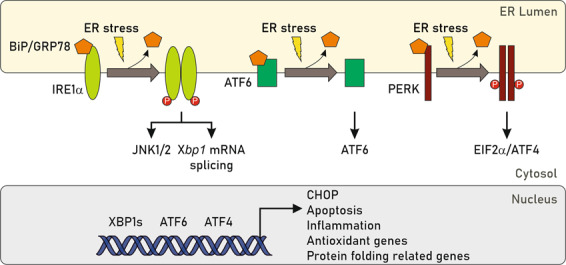
ER stress signalling involves three main protein sensors, PRKR-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme-1α (IRE1α) and activating transcription factor-6 (ATF6). These proteins remain inactive while are bound to the intraluminal chaperone glucose-regulated protein 78 (GRP78), also named Binding Protein (BiP). In response to ER stress, these mediators become activated and released, thereby triggering molecular cascades that activate the unfolded protein response (UPR). Overall, activation of each sensor promotes ATF4, XBP1s and ATF6 translocation to the nucleus to induce the expression of their relevant target genes associated with apoptosis, inflammation, antioxidant response and protein folding mechanisms, among others, to restore ER homeostasis.
