Abstract
Objective
We investigated the effect of icariin (ICA) combined with Panax notoginseng saponins (PNS) on intestinal microbiota and hippocampal protein expression in amyloid precursor protein/presenilin 1 (APP/PS1) transgenic mice, a model of Alzheimer’s disease (AD).
Methods
Transgenic mice were treated with icariin and PNS. The Morris water maze (MWM) was used to assess spatial memory, and the gut microbiota and differential protein expression in the hippocampus were investigated using high-throughput screening techniques. Differential protein expression was confirmed by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting.
Results
The MWM results showed that the mice treated with the medium dose of ICA+PNS spent significantly more time in the target quadrant compared with the AD group. Bacterial diversity was the lowest in the AD group, with significantly greater diversity in the ICA + PNS treatment group. Three proteins were selected for proteomic analysis, and qRT-PCR and Western blot were used to detect the expression of 2ʹ-5ʹ-oligoadenylate synthetase ubiquitin like 1 (Oasl1), trichoplein keratin filament-binding protein (TCHP), and tumor necrosis factor receptor associated 3-interacting protein 1 (MIPT3). Compared with control mice, MIPT3 expression was increased and Oasl1 and TCHP were reduced in the AD group. These abnormal protein expressions tended to normalization after treatment with medium dose of ICA and PNS.
Conclusion
Treatment with ICA and PNS ameliorated memory impairment in an AD mouse model. The mechanisms may be related to modulation of the intestinal microbiota and expression of Oasl1, TCHP, and MIPT3.
Keywords: Alzheimer’s disease, icariin, Panax notoginsenoside, gut microflora, proteomics, Morris water maze
Introduction
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease characterized by progressive memory loss, cognitive dysfunction, inattention, emotional disorder, and personality changes.1 AD pathogenesis is closely related to age and genetic and environmental factors.2 It is not easily diagnosed in the early stage, as the etiology and pathogenesis of AD are still not fully understood.2 The primary pathological features of AD are β-amyloid (Aβ) protein deposition in the cerebral cortex and hippocampus that form senile plaques and neurofibrillary tangles of hyperphosphorylated that damage neurons and reduce levels of choline acetyltransferase and acetylcholinesterase.3
Gut microflora (GM) refers to the microorganisms that normally populate the digestive tract. GM disorder is related to a variety of diseases.4,5 For example, the GM of AD patients and AD model mice are different from corresponding healthy controls, and the GM can participate in AD onset and development through several mechanisms.6 GM dysfunction is associated with obesity, decreased antioxidant capacity, and aging, all of which are risk factors for AD. Additionally, neurotransmitters or neurotoxic substances produced by bacteria can enter the brain through the circulatory system and affect neuronal function.7 For these reasons, GM is an interesting research focus to study AD pathogenesis.8
Traditional Chinese medicine (TCM) has made substantial progress in the treatment of AD;9 several active substances used in TCM have a certain curative effect on AD with negligible side effects.10 The main effects of TCM in AD treatment are generally based on two aspects: one is to improve symptoms with resuscitation, blood-activating, stasis-removing, and anti-aging drugs; the other is to use tonic and conditioning drugs to enhance the immune and cardiovascular systems.11,12 Icariin (ICA) from Horny Goat Weed or Yin Yang Huo has anti-aging functions of removing blood stasis and promoting blood circulation.13 Panax notoginseng saponins (PNS) are active components of Panax pseudo-ginseng and enhance immunity.14 ICA and PNS are the major active components of Yizhijiannao granules, which have documented to improve memory in AD.15,16 However, the underlying mechanisms of these compounds are still largely unknown.
In this study, ICA and PNS were used to treat amyloid precursor protein/presenilin 1 (APP/PS1) transgenic mice, followed by high-throughput detection of the intestinal GM and hippocampal proteins. The results shed light onto AD pathogenesis and provide evidence for the application of ICA and PNS in the treatment of AD.
Materials and Methods
Experimental Animals
Six-month-old male APP/PS1 transgenic mice and wild-type male mice with a similar background (C57) and age were purchased from Changzhou Cavens Experimental Animal Co., Ltd.: SCXK (Su) 2016–0010.17,18 The animals were raised in a specific pathogen-free grade environment, and the diet was strictly sterilized. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the Ministry of Science and Technology of China (2006) and that these experiments were approved by the Ethics Committee of Hunan University of Chinese Medicine.
Donepezil was used as a positive control drug. ICA and PNS were both used to treat AD mice. The animals were divided into six groups (each n=6): normal C57 mice (control), AD mice, AD + low dose of ICA+PNS, AD + medium dose of ICA+PNS, AD + high dose of ICA+PNS group, and AD + donepezil. Mice in the AD + low dose of ICA+PNS group received oral ICA at 30 mg/kg/d (F1806075, Aladdin, Shanghai, China) and PNS at 100 mg/kg/d (1019C021, Solarbio, Beijing, China) for 4 weeks. Mice in the AD + medium dose of ICA+PNS group received doses of 80 mg/kg/d ICA and 150 mg/kg/d PNS, while the corresponding high-dose group doses were 120 mg/kg/d and 200 mg/kg/d for 4 weeks. Mice in the AD + donepezil received an oral dose of 1.30 mg/kg/d for 4 weeks (F1510007, Aladdin). In the control and AD groups, C57 mice and AD mice orally received an equal volume of water. After the 4-week treatment, memory was tested with the Morris water Morris maze (MWM). After behavioral testing, mice were anesthetized with 5% isoflurane and decapitated. The intestinal contents and hippocampal tissues were collected for gut microbiota analysis and protein expression measurements, respectively.
MWM
After treatment, spatial memory was tested using the MWM as previously described.19 Briefly, a 5-day navigation experiment was carried out followed by memory testing on the sixth day. The time spent around the platform quadrant was recorded and calculated by Ugo Basile software (Ugo Basile, Gemonia, Italy).
GM Diversity Analysis
Total RNA was extracted from intestinal contents, and RNA purity was spectrophotometrically quantified. Target sequences that reflect GM composition and diversity were selected, and corresponding primers were designed according to the conservative region of the sequence to carry out polymerase chain reaction (PCR) amplification. The amplified products were detected by 2% agarose gel electrophoresis, and the target fragments were recovered by gel extraction. PCR was amplified by fluorescence quantification and then sequenced for high-throughput sequencing. Using QIIME software and the UCLUST sequence alignment tool, the sequences obtained from high-throughput sequencing were merged and divided into different operational taxonomic units (OTUs) according to 97% sequence similarity. The sequence with the highest abundance in each OTU was selected as the representative sequence of the OTU, and then the classification and identification results were obtained based on the ribosomal database project database.
Proteomic Analysis
Total proteins were obtained by centrifuging the supernatants of cell lysis, following by bicinchoninic acid (BCA) assays to quantify protein levels. Next, 100 μg protein from each sample was processed by filter-aided sample preparation, trypsin was used to degrade the samples to obtain crude peptides, and a C18 column was used for purification. Briefly, 100 μg peptide segments were taken from each sample and labeled according to the TMT labeling kit instructions (Thermo Fisher Scientific, Waltham, MA, USA). After labeling, all samples were mixed in equal amounts, and the peptide segment solution was subjected to liquid chromatography with tandem mass spectrometry online detection. MaxQuant software was used to carry out the database retrieval (https://www.uniprot.org/). Based on the quantitative results, differentially expressed proteins were identified using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
Fluorescence Quantitative Real-Time PCR (qRT-PCR)
After RNA extraction, cDNA was synthesized using a reverse transcription kit according to the manufacturer’s instructions (Dalian Baosheng, Dalian, China). The expression levels of 2ʹ-5ʹoligoadenylate synthetase ubiquitin like 1 (Oasl1), trichoplein keratin filament-binding protein (TCHP), and tumor necrosis factor receptor associated 3-interacting protein 1 (MIPT3) in each group were detected by real-time fluorescence qRT-PCR using cDNA as template. The primer sequences are listed in Table 1. The PCR samples were processed with a reaction system including 9.5 μL RNase-free distilled H2O, 1 μL cDNA/DNA, 2 μL primers, and 12.5 μL 2x ULtraSYBR Mixture using the following protocol: denaturation at 95°C for 10 s, annealing at 58.5°C for 30 s, and extension at 72°C for 30 s for 40 cycles.
Table 1.
Primer Sequences
| Primer Name | Primer Sequence (5ʹ-3ʹ) | Primer Length (bp) | Product Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| OASL1 F | GCAGACCCCACCAACAAT | 18 | 334 | 58.5 |
| OASL1 R | TTCCGTAGTAGGCGAGCGT | 19 | ||
| TCHP F | GGAGGTGATGGAGAATGCGG | 20 | 271 | 61 |
| TCHP R | CCCTGACGGCACCATGTCAA | 20 | ||
| MIPT3 F | CAGCCTCAGAAGAATACCTCG | 21 | 175 | 58.3 |
| MIPT3 R | CACCACAAACTGCTCGTCAT | 20 | ||
| GAPDH F | TCAACGGCACAGTCAAGG | 18 | 357 | 56.8 |
| GAPDH R | TGAGCCCTTCCACGATG | 17 |
Western Blot
Protein was extracted from the hippocampal tissues for Western blot analysis using Radioimmunoprecipitation Cell Lysate (C1053, Applygen, Beijing, China). Protein concentrations were determined using the BCA method. Equal amounts of protein were processed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were then transferred to polyvinylidene fluoride membranes, and the nonspecific binding was blocked by incubating in 5% skim milk for 30 min at room temperature. The membrane was incubated with primary antibodies including rabbit polyclonal anti-TCHP (1/500, OM118476, OmnimAbs, Alhambra, CA, USA), rabbit polyclonal anti-MIPT3 (1/200, DF7181, Affinity Biosciences, Cincinnati, OH, USA) and rabbit polyclonal anti-Oasl1 (1/500, ab116220; Abcam, Cambridge, UK) overnight at 4°C.
Statistical Analysis
SPSS 19 software (IBM Corp., Armonk, NY USA) was used to analyze the data, which are expressed as mean ± standard deviation. One-way analysis of variance followed by Bonferroni was used for comparison among three or more groups.
Results
ICA and PNS Ameliorated Memory Impairment in AD Mice
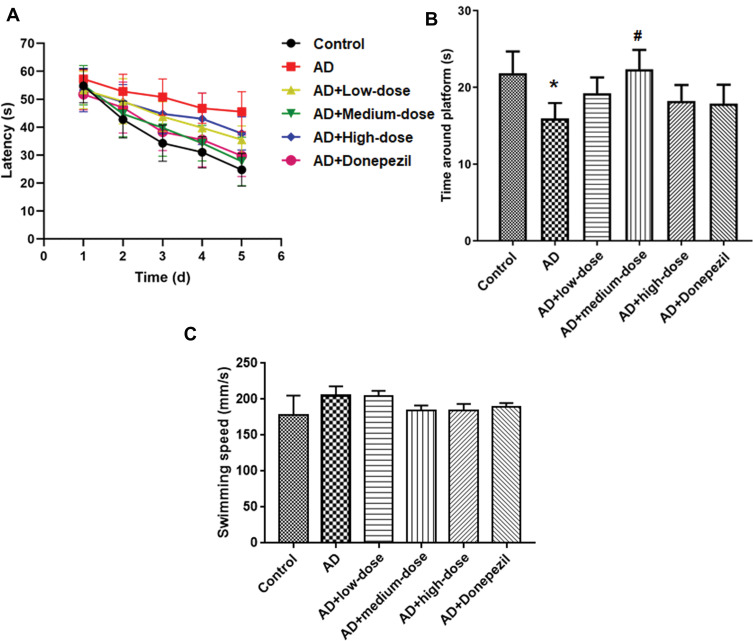
The MWM was used to test the spatial memory after treatment with three doses of ICA and PNS. As shown in Figure 1A, the time to find the platform was reduced after 5 days of training in the control mice but not in AD mice (The fifth day: control vs AD, P<0.05). Treatment with different doses of ICA and PNS promoted learning of the platform location, especially the medium dose of ICA and PNS (medium-dose vs AD, P<0.05). The time spent in the target quadrant was analyzed on the sixth day after removing the platform. The time around the platform was significantly lower in the model group, but it was increased by treatment with the medium dose of ICA and PNS (medium-dose vs AD, P<0.05) (Figure 1B). We also recorded the average swimming speeds of the animals during MWM testing, which were comparable (Figure 1C), indicating normal motor function in all groups.
Figure 1.
Treatment with ICA and PNS ameliorated memory impairment in AD mice. (A) Latency to find the platform; (B) Time in the target quadrant; (C) Swimming speeding. N=6 animals in each group. *P < 0.05 compared the control group, #P < 0.05 compared with the AD group.
Effects of ICA and PNS on GM Diversity in AD Mice
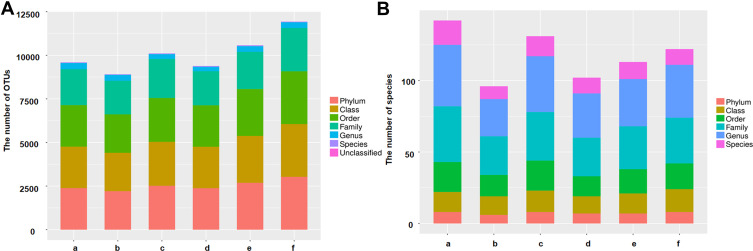
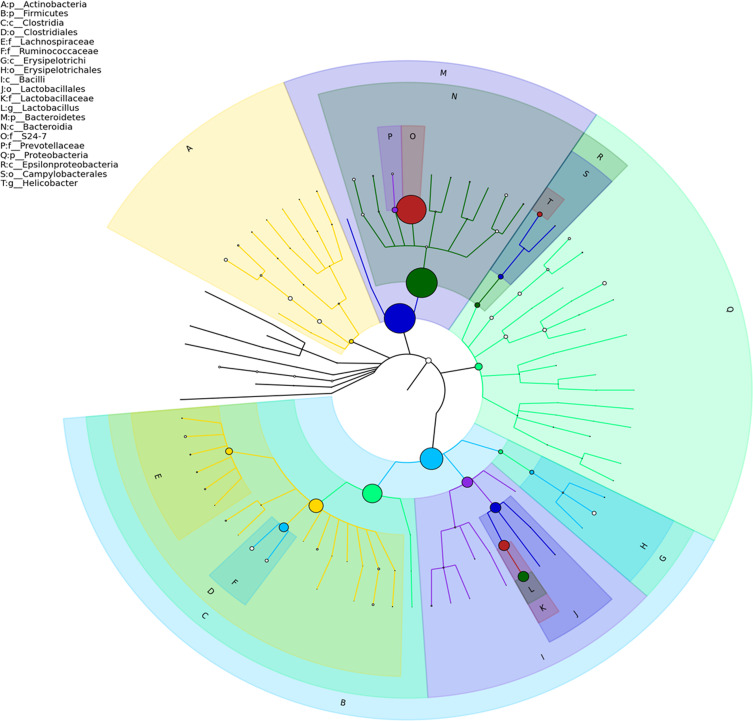
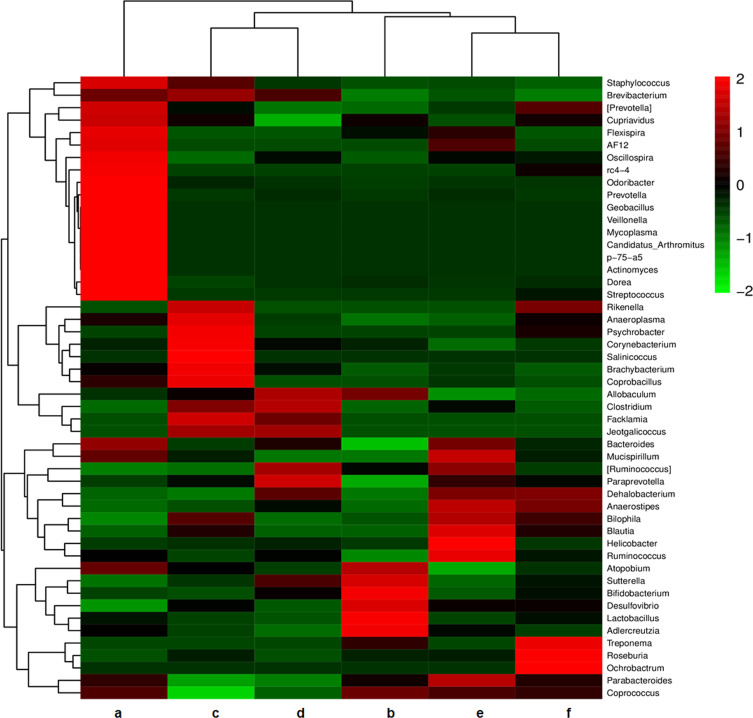
As shown in Figure 2A, the number of OTUs was lowest in the AD group, but higher numbers were observed in all three treatment groups. The number of species including Phylum, Class, Order, Family, Genus, and Species were also reduced in the AD group but increased in the ICA and PNS treatment groups (Figure 2B). Figure 3 shows the general classification tree of the samples. The highest horizontal abundance was for Firmicutes and Bacteroidetes, while the highest horizontal abundances of the Genus were for Lactobacillus and Helicobacter. Figure 4 shows the abundance heat map after clustering analysis of the first 50 genera. After treatment, the major GM components from the medium- and high-dose ICA and PNS groups and the donepezil groups were similar to the control group, indicating that ICA and PNS exerted an effect similar to the positive control drug.
Figure 2.
Treatment with ICA and PNS improved microbiota diversity in AD mice. (A) The number of OTUs; (B) the number of species. N=6 in each group. a, control; b, AD; c, AD + low-dose; d, AD + medium-dose; e, AD + high-dose; f, AD + donepezil.
Figure 3.
General classification tree of the samples.
Figure 4.
Heat map of community composition at genus level combined with cluster analysis. Red and green represent genera with higher and lower abundance, respectively. a, control; b, AD; c, AD + low-dose; d, AD + medium-dose; e, AD + high-dose; f, AD + donepezil.
Proteomic Analysis of Hippocampal Proteins
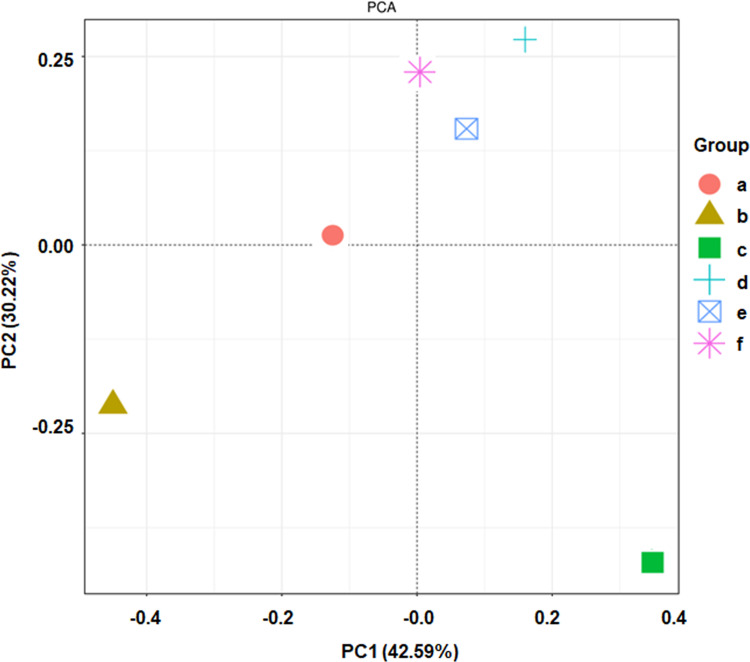
We used MaxQuant software to search the KEGG database and select three relevant differentially expressed proteins: Oasl1, TCHP, and MIPT3 (Table 2). Figure 5 shows the principal component analysis (PCA) results of Oasl1, TCHP and MIPT3 among the components.
Table 2.
Relative Expression of Selected Differential Proteins in Each Group
| Accession (Mouse) | Significance | −10lgP | Control | AD | Low-Dose | Medium-Dose | High-Dose | Donepezil |
|---|---|---|---|---|---|---|---|---|
| OASL1 | 81.17 | 24.64 | 7.04E+05 | 6.90E+04 | 2.70E+04 | 3.86E+04 | 6.91E+04 | 5.81E+05 |
| TCHP | 34.94 | 28.07 | 1.24E+05 | 4.72E+04 | 4.02E+04 | 5.07E+04 | 5.01E+04 | 1.73E+05 |
| MIPT3 | 33.05 | 33.49 | 1.52E+05 | 4.94E+04 | 3.11E+04 | 3.05E+04 | 5.96E+04 | 1.54E+05 |
Figure 5.
Two-dimensional sorting diagram of samples for PCA analysis. N=6 in each group. a, control; b, AD; c, AD + low-dose; d, AD + medium-dose; e, AD + high-dose; f, AD + donepezil.
ICA and PNS Reduced MIPT3 and Increased Oasl1 and TCHP Expression
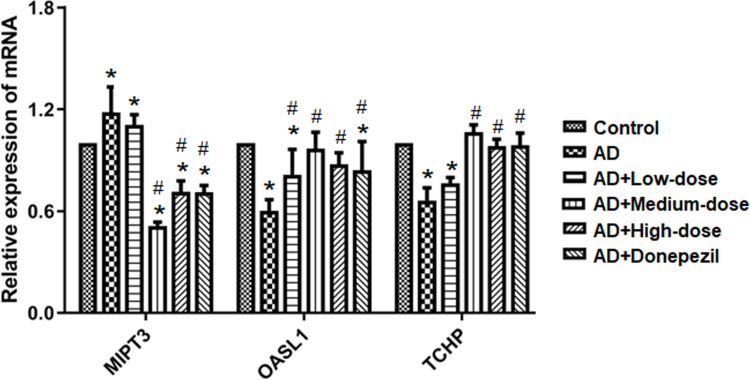
As shown in Figure 6, qRT-PCR was performed to measure mRNA expression of MIPT3, Oasl1, and TCHP. Levels of MIPT3 in the AD group were significantly higher than in the control group, while Oasl1 and TCHP in the AD group were significantly lower than in the control group (control vs AD, P<0.05). After treatment, MIPT3 levels in the medium- and high-dose groups were significantly lower than that in the AD group (vs AD, P<0.05), while Oasl1 and TCHP in the medium- and high-dose groups were significantly higher than that in the AD group (vs AD, P<0.05).
Figure 6.
Treatment with ICA and PNS reduced MIPT3 and increased Oasl1 and TCHP mRNA levels. N=6 in each group. *P < 0.05 compared the control group, #P < 0.05 compared with the AD group.
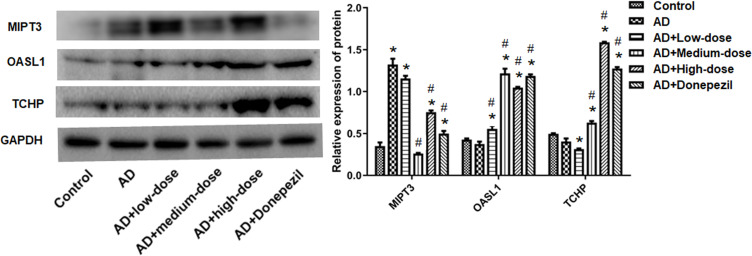
Western blotting was used to detect the expression of MIPT3, Oasl1, and TCHP (Figure 7). MIPT3 expression was significantly higher in the AD group compared to the control group (control vs AD, P<0.05), and that in the middle dose group was significantly lower than the AD group (vs AD, P<0.05). Levels of Oasl1 and TCHP in the AD group were comparable with those in the control group. However, medium and high doses of ICA and PNS significantly elevated Oasl1 and TCHP expression (vs AD, P<0.05).
Figure 7.
Treatment with ICA and PNS reduced MIPT3 and increased Oasl1 and TCHP protein levels. N=6 in each group. *P < 0.05 compared the control group, #P < 0.05 compared with the AD group.
Discussion
The drugs currently used in the clinical treatment of AD are mainly focused on symptom elimination, such as donepezil.20 These treatments can only improve or alleviate the symptoms of AD patients to a certain extent, and patients may suffer from serious adverse effects including insomnia and nausea.21 ICA has been isolated from several species of plants belonging to the genus Epimedium, which are commonly known as Horny Goat Weed or Yin Yang Huo. Its main effects are to improve cardiovascular and cerebrovascular system function, enhance immunity, and regulate the endocrine system.13 PNS are composed of ginsenosides and notoginseng saponins. PNS is the main active component of Panax notoginseng, which was able to improve cardiovascular system function and regulates immunity.22,23 In this study, AD mice treated with ICA and PNS successfully completed MWM testing, and the learning and memory of mice in the middle dose group were improved to a certain extent. These results are consistent with previous publications regarding the combined application of ICA and PNS to treat memory impairment.15,16
Learning and memory impairment are key characteristics of AD.17 Learning is a process of knowledge acquisition, while memory is the recall and expression of what we have acquired and learned.24 Both are important steps for cognition. Our results showed that both of learning and memory were impaired in AD mice. The medium dose of ICA and PNS showed a tendency improve learning and significantly recovered memory. ICA and PNS are two compounds with different structures. When administered together, the two drugs might affect different mechanisms to regulate protein expression and behavior. Dose-dependent effects were not seen, possibly because two drugs were used in combination. In can be difficult to observe a clear dose-dependent effect when evaluating multiple components or active components from herbal medicine.25 Surprisingly, the selected dose of donepezil did not promote learning and memory as measured with the MWM. This dose of donepezil may not be sufficient for this specific AD model.
GM plays important roles in AD development.26 Recent research has shown that GM abnormalities can accelerate AD progression,27 and modulating the GM provides new opportunities for preventing and treating AD.28 Brain-gut-microbiota axis dysfunction contributes to the pathogeneses of neurodegenerative disorders.29 Our results revealed that the abundances of Lactobacillus, Bifidobacterium, and Adlercreutzia were higher in the intestinal tract of AD model mice compared to the normal group, while that of Bacteroides and Paraprevotella were significantly lower. PCA of the six groups showed that after administration of ICA and PNS, the GM composition tended to the return to normal, especially for the middle-dose group. Together with the behavioral tests, these results suggest that ICA and PNS treatment may have ameliorated memory impairment through regulating GM alterations.
PCA in each group identified three proteins that were significantly differentially expressed and are related to AD: MIPT3, Oasl1, and TCHP. MIPT3 is a ciliary protein that may be involved in the transport and assembly of synthesized ciliary proteins in the cytoplasm.30 Oasl1 is a member of the 2ʹ-5ʹoligoadenylate synthetase family and has antiviral activity.31 TCHP is a tumor suppressor that can inhibit cell growth and be pro-apoptotic during cell stress. It inhibits bladder and prostate cancer cell growth by inhibiting the phosphorylation of heat shock protein family B member 1. TCHP may act as a “capping” or “branching” protein for keratin filaments at the cell periphery, and it can regulate K8/K18 filament and desmosome organization in the apical or peripheral regions of simple epithelial cells.32 The qRT-PCR and Western blot results showed that MIPT3, Oasl1, and TCHP levels in AD mice tended to normalize after combined treatment with ICA and PNS, suggested that both compounds played roles in downregulating MIPT3 and upregulating Oasl1 and TCHP. Some studies have shown that MIPT3 can negatively regulate nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) signaling pathways and positively regulate type I interferon production. MIPT3 may also negatively regulate the activity of calmodulin phosphatase. It can be speculated that ICA and PNS may regulate NF-κB and MAPK signaling pathways and inhibit the release of inflammatory factors by downregulating the expression of MIPT3, to alleviate AD symptoms.33
Oasl1 has an antiviral function that depends on its N-terminal double-stranded RNA binding domain and two ubiquitin-like regions in its C-terminus. Together, these features can activate the retinoic acid-inducible gene-I like receptor (RLR).34 In the cytoplasm, the ubiquitin-like region of Oasl1 can bind to the N-terminal caspase activation and recruitment domains (CARD) of RLR. Interferon is produced by activating interferon regulatory factor 3. Interferon further releases downstream molecules to play an antiviral role.34 TCHP may have the function of inhibiting senile plaque formation and clearing Aβ protein.35 However, this function was not been verified in this present study.
There is a close relationship between the functions of the GM and MIPT3.36 GM dysfunction could contribute to the inflammation reaction, which might be MIPT3 dependent. We did not assess the relationship between GM dysfunction and Oasl1 or TCHP. The regulatory mechanisms might be interesting, as they could clarify the pathogenesis of AD. In this study, three different doses of ICA and PNS were selected. The medium dose led to the most improvement in the behavioral test and normalized MIPT3, OASL1 and TCHP levels. These consistent results indicate that ICA and PNS might exert their effects through regulating MIPT3, OASL1, and TCHP expression. Nevertheless, we did not consider the ratio of ICA and PNS based on the original ratio described for TCM. Optimizing the ICA: PNS ratio might enhance the beneficial effects.
Collectively, our results indicate that treatment with ICA and PNS could ameliorate memory impairment in an AD mouse model. The mechanisms may be related modulation of the intestinal microbiota distribution and expression of Oasl1, TCHP, and MIPT3.
Data Sharing Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.
Disclosure
All authors declare no personal or financial conflicts of interest for this work.
References
- 1.Hoang CL, Ha GH, Pham KTH, et al. Global mapping of interventions to improve quality of life of patients with Alzheimer’s disease during 1990–2018. Dement Geriatr Cogn Disord. 2020;48(5):1–13. [DOI] [PubMed] [Google Scholar]
- 2.Sims R, Hill M, Williams J. The multiplex model of the genetics of Alzheimer’s disease. Nat Neurosci. 2020;1–12. [DOI] [PubMed] [Google Scholar]
- 3.Fan L, Mao C, Hu X, et al. New Insights Into the pathogenesis of Alzheimer’s disease. Front Neurol. 2020;10:1312. doi: 10.3389/fneur.2019.01312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma XH, Gao Q, Jia Z, Zhang ZW. Neuroprotective capabilities of TSA against cerebral ischemia/reperfusion injury via PI3K/Akt signaling pathway in rats. Int J Neurosci. 2015;125(2):140–146. doi: 10.3109/00207454.2014.912217 [DOI] [PubMed] [Google Scholar]
- 5.Quigley EM. Gut bacteria in health and disease. Gastroenterol Hepatol. 2013;9(9):560–569. [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis. 2017;58(1):1–15. doi: 10.3233/JAD-161141 [DOI] [PubMed] [Google Scholar]
- 7.Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333. doi: 10.1126/sciadv.aau3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguayo S, Schuh C, Vicente B, Aguayo LG. Association between Alzheimer’s disease and oral and gut microbiota: are pore forming proteins the missing link? J Alzheimers Dis. 2018;65(1):29–46. [DOI] [PubMed] [Google Scholar]
- 9.Howes MR, Fang R, Houghton PJ. Effect of Chinese herbal medicine on Alzheimer’s disease. Int Rev Neurobiol. 2017;135:29–56. [DOI] [PubMed] [Google Scholar]
- 10.Yang WT, Zheng XW, Chen S, et al. Chinese herbal medicine for Alzheimer’s disease: clinical evidence and possible mechanism of neurogenesis. Biochem Pharmacol. 2017;141:143–155. doi: 10.1016/j.bcp.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Cheng X, Huang Y, Zhang Y, Zhou W. LW-AFC, a new formula from the traditional Chinese medicine Liuwei Dihuang decoction, as a promising therapy for Alzheimer’s disease: pharmacological effects and mechanisms. Adv Pharmacol. 2020;87:159–177. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Wang X, Xie L, et al. Extract of Danggui-Shaoyao-San ameliorates cognition deficits by regulating DHA metabolism in APP/PS1 mice. J Ethnopharmacol. 2020;253:112673. doi: 10.1016/j.jep.2020.112673 [DOI] [PubMed] [Google Scholar]
- 13.Zhu WL, Zheng JY, Cai WW, et al. Ligustilide improves aging-induced memory deficit by regulating mitochondrial related inflammation in SAMP8 mice. Aging. 2020;12(4):3175–3189. doi: 10.18632/aging.102793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei JR, Wen X, Bible PW, Li Z, Nussenblatt RB, Wei L. Panax notoginseng saponin controls IL-17 expression in helper T cells. J Ocul Pharmacol Ther. 2017;33(4):285–289. doi: 10.1089/jop.2016.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T, Zhang Z, Dong K, Li G, Zhu H. Yizhijiannao granule and a combination of its effective monomers, icariin and panax notoginseng saponins, inhibit early PC12 cell apoptosis induced by beta-amyloid (25–35). Neural Regen Res. 2012;7(24):1845–1850. doi: 10.3969/j.issn.1673-5374.2012.24.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng M, Qu L, Lou Y. Effects of icariin combined with panax notoginseng saponins on ischemia reperfusion-induced cognitive impairments related with oxidative stress and CA1 of hippocampal neurons in rat. Phytother Res. 2008;22(5):597–604. doi: 10.1002/ptr.2276 [DOI] [PubMed] [Google Scholar]
- 17.Zhu G, Yang S, Xie Z, Wan X. Synaptic modification by L-theanine, a natural constituent in green tea, rescues the impairment of hippocampal long-term potentiation and memory in AD mice. Neuropharmacology. 2018;138:331–340. doi: 10.1016/j.neuropharm.2018.06.030 [DOI] [PubMed] [Google Scholar]
- 18.Hong X, Liu J, Zhu G, et al. Parkin overexpression ameliorates hippocampal long-term potentiation and beta-amyloid load in an Alzheimer’s disease mouse model. Hum Mol Genet. 2014;23(4):1056–1072. doi: 10.1093/hmg/ddt501 [DOI] [PubMed] [Google Scholar]
- 19.Amini M, Ma CL, Farazifard R, et al. Conditional disruption of calpain in the CNS alters dendrite morphology, impairs LTP, and promotes neuronal survival following injury. J Neurosci. 2013;33(13):5773–5784. doi: 10.1523/JNEUROSCI.4247-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong-Qi Y, Zhi-Kun S, Sheng-Di C. Current advances in the treatment of Alzheimer’s disease: focused on considerations targeting Abeta and tau. Transl Neurodegener. 2012;1(1):21. doi: 10.1186/2047-9158-1-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briggs R, Kennelly SP, O’Neill D. Drug treatments in Alzheimer’s disease. Clin Med. 2016;16(3):247–253. doi: 10.7861/clinmedicine.16-3-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang JW, Du YQ, Li CJ, et al. Neuroprotective triterpene saponins from the leaves of Panax notoginseng. Nat Prod Res. 2019;1–7. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Xue R, Li L, et al. Panax notoginseng saponins protect cardiac myocytes against endoplasmic reticulum stress and associated apoptosis through mediation of intracellular calcium homeostasis. Front Pharmacol. 2019;10:1013. doi: 10.3389/fphar.2019.01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Z, Chen H, Xu W, Wu S, Zhu G. Basolateral amygdala calpain is required for extinction of contextual fear-memory. Neurobiol Learn Mem. 2018;155:180–188. doi: 10.1016/j.nlm.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 25.Yang SJ, Song ZJ, Wang XC, Zhang ZR, Wu SB, Zhu GQ. Curculigoside facilitates fear extinction and prevents depression-like behaviors in a mouse learned helplessness model through increasing hippocampal BDNF. Acta Pharmacol Sin. 2019;40(10):1269–1278. doi: 10.1038/s41401-019-0238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu X, Wang T, Jin F. Alzheimer’s disease and gut microbiota. Sci China Life Sci. 2016;59(10):1006–1023. [DOI] [PubMed] [Google Scholar]
- 27.Giau VV, Wu SY, Jamerlan A, An SSA, Kim SY, Hulme J. Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients. 2018;10:11. doi: 10.3390/nu10111765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Sun G, Feng T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019;29(10):787–803. doi: 10.1038/s41422-019-0216-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowalski K, Mulak A. Brain-gut-microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil. 2019;25(1):48–60. doi: 10.5056/jnm18087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng MH, Ho TH, Kok KH, Siu KL, Li J, Jin DY. MIP-T3 is a negative regulator of innate type I IFN response. J Immunol. 2011;187(12):6473–6482. doi: 10.4049/jimmunol.1100719 [DOI] [PubMed] [Google Scholar]
- 31.Hovanessian AG, Justesen J. The human 2ʹ-5ʹoligoadenylate synthetase family: unique interferon-inducible enzymes catalyzing 2ʹ-5ʹ instead of 3ʹ-5ʹ phosphodiester bond formation. Biochimie. 2007;89(6–7):779–788. doi: 10.1016/j.biochi.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 32.Inoko A, Matsuyama M, Goto H, et al. Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol. 2012;197(3):391–405. doi: 10.1083/jcb.201106101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu Y, Murata T, Watanabe K, et al. MIP-T3 associates with IL-13Ralpha1 and suppresses STAT6 activation in response to IL-13 stimulation. FEBS Lett. 2003;550(1–3):139–143. doi: 10.1016/S0014-5793(03)00860-3 [DOI] [PubMed] [Google Scholar]
- 34.Choi BY, Sim CK, Cho YS, et al. 2ʹ-5ʹ oligoadenylate synthetase-like 1 (OASL1) deficiency suppresses central nervous system damage in a murine MOG-induced multiple sclerosis model. Neurosci Lett. 2016;628:78–84. doi: 10.1016/j.neulet.2016.06.026 [DOI] [PubMed] [Google Scholar]
- 35.Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta. 2006;1757(5–6):692–699. doi: 10.1016/j.bbabio.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 36.Nimmo ER, Stevens C, Phillips AM, et al. TLE1 modifies the effects of NOD2 in the pathogenesis of Crohn’s disease. Gastroenterology. 2011;141(3):972–981 e971–972. doi: 10.1053/j.gastro.2011.05.043 [DOI] [PubMed] [Google Scholar]