FIGURE 1.
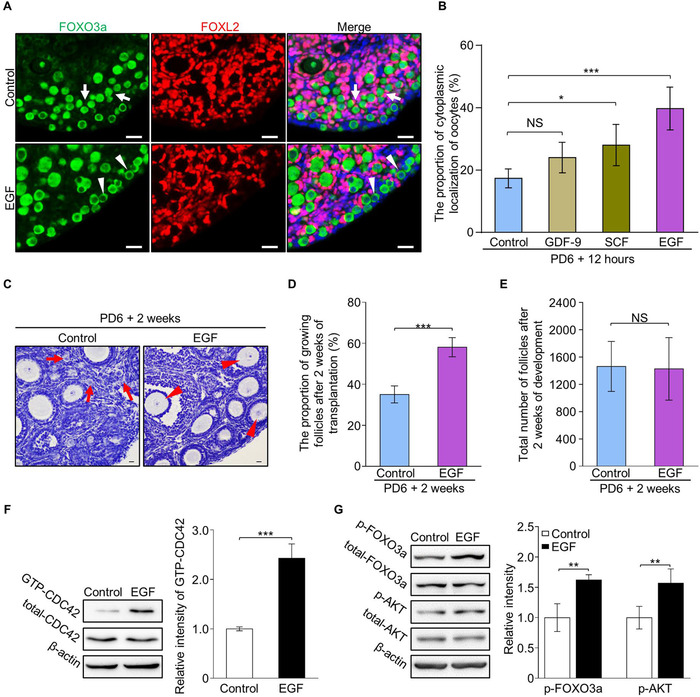
EGF treatment improves the activation of primordial follicles by elevating CDC42‐PI3K signaling in mouse ovaries. (A) FOXO3a (green) shuttled from the nuclei (arrows) to the cytoplasm (arrowheads) with oocyte activation. EGF treatment for 30 minutes markedly increased cytoplasmic localization of FOXO3a (arrowheads) in oocytes compared to controls. Granulosa cells were dyed with FOXL2 antibody (purple) to show the follicles and nuclei were dyed with a Hoechst counterstain (blue). (n = 6). (B) Quantification results revealed a significantly increased ratio of cytoplasm localization of FOXO3a in the oocytes of EGF (40 ± 6%), GDF‐9 (24 ± 4%) and SCF (28 ± 6%) treated ovaries compared to vehicle (17 ± 3%) treated ovaries (n > 3). (C) After 2 weeks of transplantation, primordial follicles in clusters (arrows) were detected in the control ovaries (n = 5) whereas an increased number of growing follicles (arrowheads) was observed in the cortical region of EGF‐treated ovaries (n = 5). (D) Follicle quantification showed a significantly increased percentage of growing follicles in the EGF group compared to controls (58 ± 4% vs 35 ± 4%) (n = 5). (E) EGF treatment had no effect on follicle survival (1428 ± 410 vs 1464 ± 372) (n = 5). (F) The CDC42‐GTP pull‐down assay showed that CDC42‐GTP levels were significantly increased in EGF‐treated ovaries compared to the controls. (G) Signaling studies in EGF‐treated and control ovaries, showing an enhanced level of p‐AKT and p‐FOXO3a in the EGF group compared to control ovaries. Levels of total FOXO3a, AKT, and β‐actin were used as internal controls. The experiments were repeated at least three times. In B, D, E, F, and G, data represent the mean ± SD of biological triplicate experiments. NS, P > .05, *P < .05, **P < .01, and ***P < .001, by two‐tailed unpaired Student's t test. Scale bars: 25 µm
