FIGURE 1.
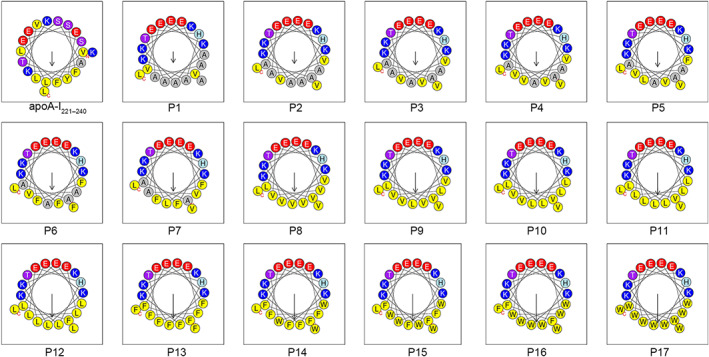
The designed peptides (P1–P17) with cholesterol efflux activities possess an amphipathic α‐helix structure (figures were exported from HeliQuest online software [https://heliquest.ipmc.cnrs.fr/]). The design strategy was as follows: First, the hydrophobic amino acids on the hydrophilic surface of apoA‐I221–240 were replaced with hydrophilic amino acids, and the hydrophilic amino acids on the hydrophobic surface of apoA‐I221–240 were replaced with hydrophobic amino acids. Subsequently, to facilitate the study of the effect of hydrophobicity on the amphiphilic apoA‐I mimetic peptides, the substituted amino acids on the hydrophilic surface were fixed, and the hydrophobic amino acids on the hydrophobic surface were gradually replaced with more hydrophobic amino acids. The difference in the hydrophobicity value between two adjacent numbered peptides was approximately 0.05
