FIGURE 2.
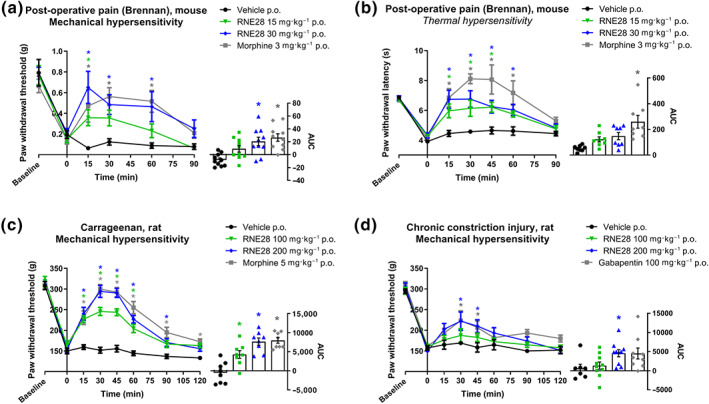
RNE28 effect on thermal and mechanical pain thresholds in inflammatory and neuropathic rodent pain models. (a) Left, time course of the antinociceptive effect induced by 3 mg·kg−1 morphine (n = 8) or 0 (n = 8), 15 (n = 8) and 30 (n = 8) mg·kg−1 RNE28 administered orally in mice 24 h following post‐operative pain induction by paw incision and suture. Pain thresholds were evaluated by the von Frey test. Right, AUCs calculated from 0 to 90 min post‐administration. (b) Left, time course of the antinociceptive effect induced by 3 mg·kg−1 morphine (n = 10) or 0 (n = 10), 15 (n = 10) or 30 (n = 10) mg·kg−1 RNE28 administered orally in mice 48 h following post‐operative pain induction by paw incision and suture. Pain thresholds were assessed by paw immersion in 46°C water. Right, AUCs calculated from 0 to 90 min post‐administration. (c) Left, time course of the antinociceptive effect induced by 5 mg·kg−1 morphine (n = 8) or 0 (n = 8), 100 (n = 8) or 200 (n = 8) mg·kg−1 RNE28 administered orally in rats 4 h following paw inflammation induction by intraplantar carrageenan injection (200 μl, 2%). Pain thresholds were assessed by the paw pressure test. Right, AUCs calculated from 0 to 120 min post‐administration. (d) Left, time course of the antinociceptive effect induced by 100 mg·kg−1 gabapentin (n = 7) or 0 (n = 9), 100 (n = 10) or 200 (n = 10) mg·kg−1 RNE28 administered orally in neuropathic rats 14 days following chronic constriction of the sciatic nerve. Pain thresholds were assessed by the paw pressure test. Right, AUCs calculated from 0 to 120 min post‐administration. * P < 0.05 versus vehicle. Two‐way ANOVA followed by Dunnett's post hoc test (a–d left) and Kruskal–Wallis followed by Dunnett's post hoc test (a–d right). n numbers represent mice (a, b) and rats (c, d)
