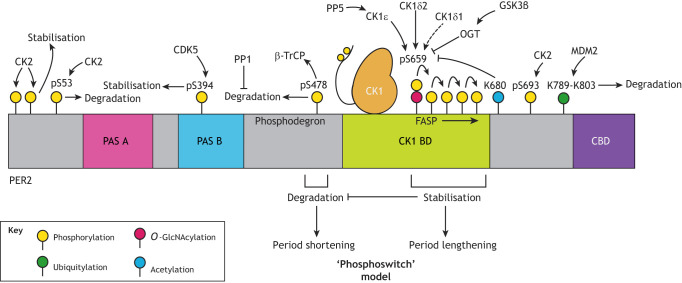
Fig. 2.
Schematic illustration of post-translational regulation of PER2. PER2 abundance and stability is a crucial determinant of circadian period and phase, with PER stabilisation associated with increased cellular period, whereas destabilisation of PER2 shortens the period. Phosphorylation of PER2 by CK1 is integral to regulation of PER2 stability. CK1δ and CK1ε bind to PER2 at the CK1-binding domain (CK1 BD) and can either stabilise or promote degradation of PER2, depending on the exact modification site, with phosphorylation at S478 in the phosphodegron promoting recruitment of the E3 ubiquitin ligase β-TrCP and thus degradation, whereas phosphorylation at the FASP region delays degradation. This interplay constitutes the ‘phosphoswitch’ model, which proposes that the balance of phosphorylation between these stabilising and degrading regions determines overall PER2 half-life. Dephosphorylation of PER2 by protein phosphatase 1 (PP1) reduces its overall degradation. In addition, CK2-mediated phosphorylation at the extreme N-terminus is proposed to stabilise the protein, whereas CK2 phosphorylation at S53 is thought to promote degradation, although more recent work suggests that the most significant site of CK2 phosphorylation on PER2 is S693. O-GlcNAcylation by O-GlcNAc transferase (OGT) at the priming serine (S659 in mice/S662 in humans) of the FASP site competitively inhibits PER2 phosphorylation by CK1, with GSK3β being a positive regulator of OGT activity. Acetylation at K680 also inhibits phosphorylation at the priming serine residue. Furthermore, ubiquitylation by MDM2 at sites downstream of FASP promotes PER2 degradation in a phosphorylation-independent manner, whereas phosphorylation at S394 within the PAS-B domain by CDK5 promotes stability. Phosphorylation and dephosphorylation (e.g. by PP5) of the C-terminal tail of CK1 can also influence its activity on its PER2 substrate. Further modifications that are discussed in the text but which currently lack a defined site of action or function, including direct phosphorylation of PER2 by GSK3β, decacetylation by SIRT1 and some O-GlcNAcylated serine residues, are not shown.