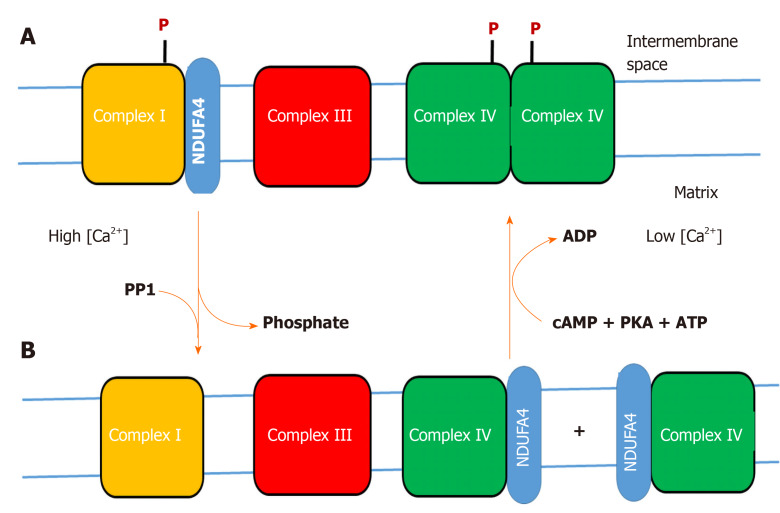
Figure 1.
Hypothesis on the variable binding of NDUFA4 to complex I or cytochrome c oxidase. A: Phosphorylation (P) of complex I and cytochrome c oxidase (CytOx) at low cytosolic calcium (< 1 micromolar) stabilizes binding of NDUFA4 to complex I and the function of the “allosteric ATP-inhibition” of dimeric CytOx (resting state); B: Stress-induced increase of cytosolic calcium (> 1 micromolar) dephosphorylates complex I and CytOx by a calcium-activated PP1, accompanied by monomerization of CytOx and switching off its allosteric ATP-inhibition. NDUFA4 is detached from complex I and binds to monomeric CytOx (excited state). At low cytosolic calcium, a cAMP-dependent protein kinase A phosphorylates complex I and CytOx. This changes the binding position of NDUFA4 from CytOx to complex I, accompanied by dimerization of CytOx and activation of its allosteric ATP-inhibition.