Summary
How does our body affect the way we think about our personality? We addressed this question by eliciting the perceptual illusion that pairs of friends swapped bodies with each other. We found that during the illusion, the participants rated their own personality characteristics more similarly to the way they previously rated their friend's personality, and this flexible adjustment of self-concept to the “new” bodily self was related to the strength of illusory ownership of the friend's body. Moreover, a subsequent memory test showed that personality traits rated during the friend-body-swap illusion were generally remembered worse than traits rated during the control conditions; importantly, however, this impairment of episodic recognition memory was reduced for the participants who considerably adjusted their self-concept during the illusory body swapping. These findings demonstrate that our beliefs about own personality are dynamically shaped by the perception of our body and that coherence between the bodily and conceptual self-representations is important for the normal encoding of episodic memories.
Subject Areas: Behavioral Neuroscience, Cognitive Neuroscience
Graphical Abstract
Highlights
-
•
We used the perceptual illusion that pairs of friends swapped bodies with each other
-
•
Each participant rated their own and their friend's personality characteristics
-
•
During the illusion, beliefs about the self and about the friend became more similar
-
•
This self-concept updating was beneficial for the ongoing memory encoding
Behavioral Neuroscience; Cognitive Neuroscience
Introduction
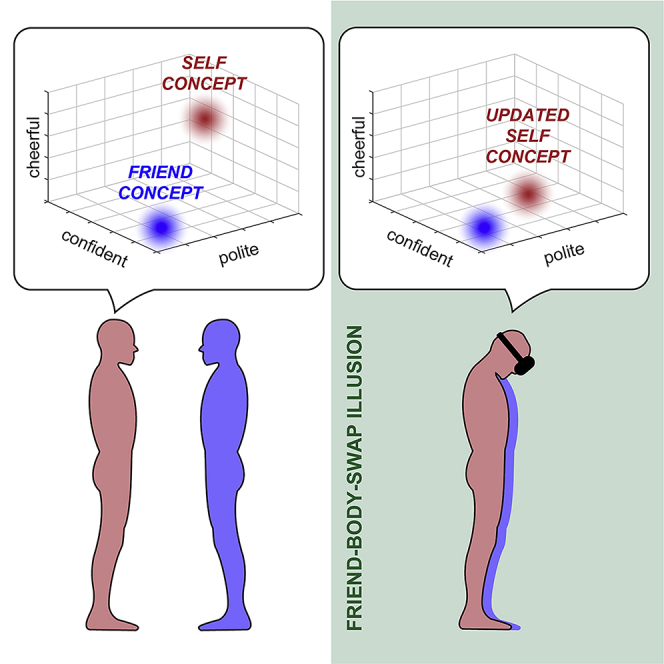
What makes us who we are? Is it the body we wake up in every morning and use as a “vehicle” through our daily activities, or is it a collection of thoughts and beliefs that we have about ourselves as individuals with certain skills, traits, and social identity? If it is a combination of the two, then how would a unified sense of self emerge from such a fusion of conscious beliefs and bodily perceptual experience? For example, would our sense of who we are change if one day our mind woke up inside the body of our best friend? These questions relate to one of the most fundamental problems in psychology and neuroscience: how we come to perceive a coherent sense of self. In addition to general relevance to all of us as thinking individuals, mechanisms of self-unity are important for the treatment of psychiatric disorders in which a sense of self is fragmented, such as in depersonalization disorder or schizophrenia.
One major element of the mental representation of ourselves is the self-concept: the multiple beliefs that we have about our own personality (Baumeister, 1998; Oyserman et al., 2012). These beliefs have a multidimensional structure that is unique for each person, and self-concept is commonly regarded as a “reference point” that organizes our experience, guides our complex behaviors, and helps to predict future situations (Baumeister, 1998; Oyserman et al., 2012; Swann, 1997). Owing to its robust representation in our memory system, self-concept also facilitates the encoding of new information; for example, trait adjectives rated in relation to the self are remembered better than traits encoded in other semantic contexts, the so-called self-reference effect (Rogers et al., 1977; Sui and Humphreys, 2015; Symons and Johnson, 1997). A second main component of our self-representation is the bodily self, that is, a sense of being distinct from the outside world and centered within a body that feels like our own (Blanke et al., 2015; Brugger and Lenggenhager, 2014; Ehrsson, 2020, 2012). Remarkably, experimental manipulations of visuotactile synchrony induce perceptual illusions that fake limbs (Botvinick and Cohen, 1998; Ehrsson et al., 2004; Tsakiris and Haggard, 2005) or even entire artificial bodies (Petkova et al., 2011; Petkova and Ehrsson, 2008) viewed from a natural first-person perspective become part of the bodily self, which demonstrates that the representation of our own body is highly flexible and that multisensory integration mechanisms play a key role in attributing ownership to our body (Ehrsson, 2012, 2020; Kilteni et al., 2015; for studies on self-recognition of bodies and faces viewed at a distance from a third-person perspective, see also Lenggenhager et al., 2007; Tsakiris, 2008; Aspell et al., 2009; Preston et al., 2015). Research has also shown that such experimentally induced changes of the bodily self have specific cognitive, emotional, and behavioral consequences; for example, attitudes toward a racial group change after the illusory embodiment of a member of that group (Maister et al., 2013; Peck et al., 2013), emotional feelings of social fear (Guterstam et al., 2015a) and body dissatisfaction (Preston and Ehrsson, 2016) can be modulated by full-body ownership illusions, and the recognition of one's own face (Sforza et al., 2010; Tajadura-Jimenez et al., 2012; Tsakiris, 2008), the style of one's own behavior (Yee et al., 2009), and implicit associations with the past self (Banakou et al., 2013) are flexibly shaped by the ongoing perception of one's own body. Importantly, experimental disruptions of a sense of the bodily self through an induction of an out-of-body illusion (Bergouignan et al., 2014) or by making the body invisible (Bréchet et al., 2019) also impair the ongoing encoding of episodic memories. What remains unknown, however, is whether the bodily self dynamically shapes multiple beliefs that constitute the conscious self-concept, and if so, what the function of this shaping is for episodic memory.
Here, we induced the perceptual illusion that pairs of friends “swapped” bodies with each other to test the hypothesis that self-concept is flexibly adjusted to the ongoing perception of one's own body and that this adjustment is beneficial for the ongoing encoding of episodic information. Based on neurocognitive models of the human self (Apps and Tsakiris, 2014; Tsakiris, 2017), evidence from social psychology (Campbell et al., 1996; Festinger, 1957; Hirsh and Kang, 2016), and studies on individuals with depersonalization disorder or schizophrenia who feel “detached” from their body (Giesbrecht et al., 2010; Postmes et al., 2014; Sierra and David, 2011), we reasoned that coherence of self-representation is functionally advantageous, whereas self-incoherence is associated with cognitive, emotional, and behavioral deficits. We theorized that this would make sense because for our self-representation to be informative in the ever-changing world, it needs to be accurate and up to date with regard to the current sensory, social, and cultural context. Thus, illusory ownership of the friend's body in our paradigm should lead to updating of the participants' beliefs about their own personality so that they become more similar to beliefs about the friend's personality. Moreover, the mismatch between the bodily and conceptual self-representations experienced during the “friend-body-swap illusion” should impair the ongoing encoding of episodic information, in line with earlier work (Bergouignan et al., 2014). Importantly, however, reinstating a coherent sense of self should reduce this memory impairment.
Results
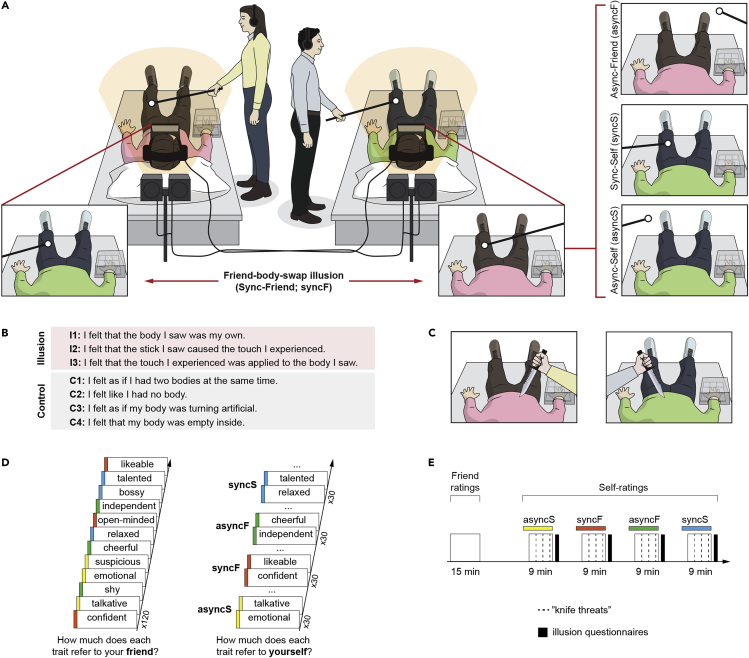
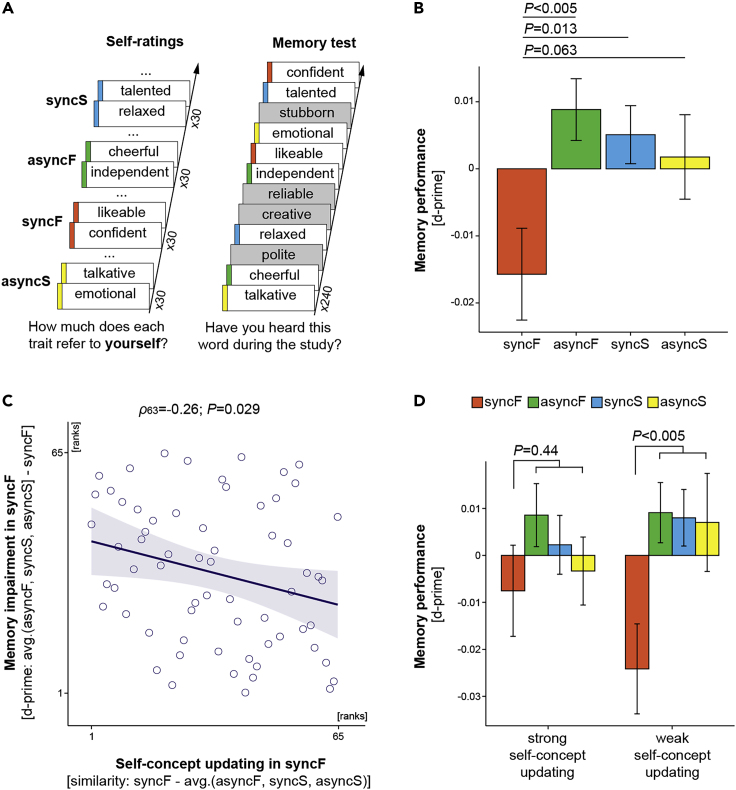
Pairs of friends participated in this study simultaneously (N = 66). During the main friend-body-swap illusion condition (“synchronous-friend”; syncF), both friends lay on beds, and through head-mounted displays (HMDs), they saw live recordings from cameras placed just above the other person's head, that is, from the perspective from which one normally looks at one's own body. At the same time, the experimenters applied synchronous touches to both participants on the corresponding body parts (Figure 1A; Video S1). A match between touches felt on one's actual body, which was out of view, and touches seen on the friend's body, which was displayed in the HMDs, should induce the perceptual illusion that the friend's body is one's own (Guterstam et al., 2015a; Petkova et al., 2011; Petkova and Ehrsson, 2008; van der Hoort et al., 2011). In contrast, asynchronous visuotactile stimulation in the “asynchronous-friend” (asyncF) condition, implemented by delaying the display in HMDs by three seconds, should reduce the illusion and serve as a well-matched control (i.e., the same visual input but no ownership of the friend's body). The “synchronous-self” (syncS) condition was a baseline built into our design; during this condition, the participants saw their own body and experienced no visuotactile delay, similar to everyday life. Finally, the “asynchronous-self” (asyncS) condition controlled for any potential effects of asynchronous visuotactile stimulation itself. The strength of the full-body ownership illusion was assessed by a questionnaire administered after each condition (Figure 1B) in which the participants rated the strength of three perceptual experiences associated with the illusion (e.g., “I felt that the body I saw was my own”) and four conceivable experiences that were not directly related to our experimental manipulation (e.g., “I felt that my body was empty inside”); the latter control items accounted for potential effects of suggestibility or task compliance (Guterstam et al., 2015a; Petkova et al., 2011; Petkova and Ehrsson, 2008; van der Hoort et al., 2011). The illusion was also assessed by skin conductance responses measured when the friend's or one's own real body was “threatened” with a mock knife (Figure 1C); this measure was intended to provide objective physiological evidence that the full-body ownership illusion was successfully induced (Guterstam et al., 2015a; Petkova et al., 2011; Petkova and Ehrsson, 2008; van der Hoort et al., 2011).
Figure 1.
Procedure
(A) Induction of the friend-body-swap illusion and visuotactile stimulation in the control conditions. The participants—a pair of friends (pink and green jumpers)—lay on two beds and wore two sets of head-mounted displays (HMDs). The recordings shown in the HMDs came from two digital cameras placed just above and behind each participant's head. This created high-quality 3D movies of either the friend's body (syncF, asyncF) or one's own body (syncS, asyncS) shown from a first-person perspective. At the same time, the experimenters applied strokes to the participants' bodies; the location, onset, and duration of each stroke were precisely controlled by audio cues heard by the experimenters. In the synchronous conditions, the touches seen in the HMDs and the touches felt on one's actual body were matched, whereas in the asynchronous conditions, the displays were delayed 3 s, creating a visuotactile mismatch.
(B) Illusion questionnaire. After each condition, the participants rated illusion (I1:I3) and control (C1:C4) statements on a 7-point scale (−3 “strongly disagree”; +3 “strongly agree”).
(C) Knife threats. Genuine ownership of the friend's body should be associated with increased physiological stress responses when this body is physically threatened. Thus, during each condition, we simultaneously “attacked” both participants' bodies with mock knives and measured skin conductance responses during these events.
(D) Friend rating and self-rating tasks. At the beginning of the study, before any body perception manipulation was applied, the participants listened to 120 trait adjectives and rated how well each trait described their friend (1 “not at all”; 9 “very much”). The same traits were then randomly assigned to the four full-body illusion conditions, and during each condition, the participants rated how well each trait described themselves.
(E) Timeline. Condition order was randomized across participants. The break between friend- and self-rating tasks was ~10 min; during this time, the full-body illusion setup was prepared.
The upper panels show what the participants are seeing in the Head Mounted Displays. For more information, please see the Results and Methods sections.
With regard to our main hypotheses, the participants performed two personality rating tasks (Figure 1D). The first friend-rating task was conducted before the four full-body illusion conditions and did not involve any body perception manipulations. During this task, the participants sat in front of computers and rated how well each of the 120 trait characteristics described the friend (Data S1). In the following self-rating task, the same traits were randomly assigned to the four full-body illusion conditions (30 traits per condition), and during each condition, the participants rated how well each trait described themselves. Ratings from these two tasks allowed us to measure how similar the participants' beliefs about their own and the friend's personalities were during different embodiment contexts (Figure 1E). Because the friend ratings in the present paradigm were “fixed” (i.e., they were collected before the four full-body illusion conditions) and because the assignment of traits to different conditions was random, an increase in similarity between self-ratings and friend ratings in a given condition, compared with other conditions, suggests an adjustment of beliefs about one's own personality to beliefs about the friend's personality in that condition (“updating of self-concept”) and not the other way around.
Eliciting the Friend-Body-Swap Illusion
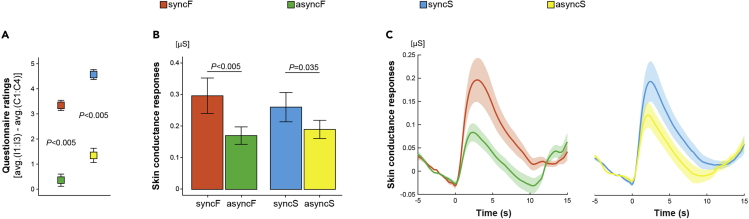
We first checked whether the friend-body-swap illusion was successfully induced. To this end, for each participant, we calculated “illusion scores” as differences between the average illusion (I1:I3) and control (C1:C4) ratings from the illusion questionnaire. These scores provided an overall estimate of the full-body illusion strength above potential suggestibility or task-compliance effects (see earlier). As expected, we found that in the synchronous conditions, the illusion scores were significantly (p < 0.005) higher than in the asynchronous conditions (Figures 2A and S1A; for detailed statistical results, see Tables S1 and S2). Moreover, and in agreement with the above results for the illusion scores, all three individual illusion statements in the syncF condition were associated with positive ratings (median ≥ +2) that were significantly (p < 0.005) higher than in the asyncF condition, which means that the majority of participants affirmed both illusory ownership and sensing touch on the friend's body in the syncF condition (Tables S3 and S4; Figure S2). Furthermore, and importantly, knife threats that occurred during the synchronous conditions evoked stronger skin conductance responses than knife threats during the asynchronous conditions (Figures 2B, 2C, and S1B and Tables S1 and S2). It is also worth noting that there was no significant modulation of the friend-body-swap illusion strength by closeness of friendship, friendship duration, participants' sex, condition order, or baseline self-friend similarity in syncS (Figure S5). Thus, both the questionnaire and skin conductance data show that the friend-body-swap illusion was successfully induced in the syncF condition and that visuotactile asynchrony in asyncS weakened the ownership of one's actual body.
Figure 2.
Synchronous Visuotactile Stimulation in syncF Successfully Induced the Friend-Body-Swap Illusion, Whereas Asynchronous Stimulation in asyncS Reduced Ownership of One's Own Actual Body
(A) Illusion scores were significantly higher in the synchronous than in the asynchronous conditions (p < 0.005). Plot shows means ± SE.
(B) Knife threats that occurred during the synchronous conditions triggered significantly stronger skin conductance responses than knife threats during the asynchronous conditions (p < 0.005). Bar plot shows means ± SE.
(C) Time courses of the skin conductance signal during knife threat events plotted for descriptive purposes. To take into account typical physiological variability of response latencies, we time-locked each response to its onset (time “0”; see Transparent Methods). Solid lines are averages of all trials, and shaded areas correspond to SE. For the detailed statistical results behind this figure, see Tables S1 and S2; for individual data points, Figure S1; and for full questionnaire results, Tables S3 and S4 and Figure S2.
Friend-Body-Swap-Induced Updating of Self-Concept
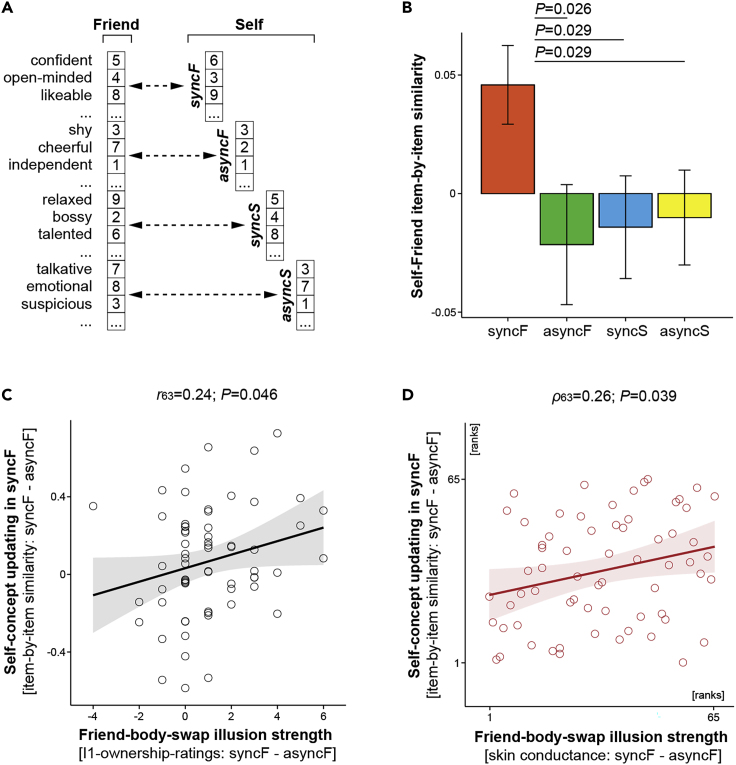
To test our first main hypothesis that the bodily self dynamically shapes the content of self-concept, for each participant in each condition, we calculated cosine similarity between self-ratings and friend ratings of the same personality characteristics (Figure 3A; Transparent Methods). Cosine similarity is a common metric of resemblance between arrays of different text items that ranges from 1 (identical) to −1 (dissimilar). To account for the overall degree of similarity between the way the participants viewed themselves and their friends, which could obviously vary across participants, we “centered” similarity scores from each condition on the average of scores from all conditions for a given participant. We found that during the friend-body-swap illusion, the participants rated themselves more similarly to the way they previously rated the friend (Figures 3B and S3A; syncF vs. asyncF: b = 0.07; SE = 0.03; t = 2.24; p = 0.026; syncF vs. syncS: b = 0.06; SE = 0.03; t = 2.22; p = 0.029; syncF vs. asyncS: b = 0.06; SE = 0.03; t = 2.21; p = 0.029; linear mixed-models [LMMs]; two-sided; N = 65). Moreover, the stronger the ownership of the friend's body during syncF, the higher the similarity between self-ratings and friend ratings in this condition. This significant correlation was present for the questionnaire and skin conductance measures of the illusion (Figures 3C and 3D, respectively; r63 = 0.24; p = 0.046; Pearson correlation; two-sided; ρ63 = 0.26; p = 0.039; Spearman correlation; two-sided; N = 65). Control analyses further excluded the possibility that increased similarity between beliefs about one's own and the friend's personalities in syncF was driven by more negative self-evaluations in this condition, a concern related to the possibility that “self-enhancement bias” (Brown, 1986) was reduced by illusory ownership of the friend's body (Figure S6). Collectively, the above results support our first main hypothesis and show that the perception of one's own body dynamically shapes multiple conscious beliefs that constitute the self-concept.
Figure 3.
Illusory Ownership of the Friend's Body in syncF Was Related to Updating of Beliefs About One's Own Personality So That They Became More Similar to Beliefs about the Friend
(A) For each participant in each condition, we calculated similarity scores between the self-ratings and friend ratings of the same traits.
(B–D) (B) During the friend-body-swap illusion, the participants rated their own personality characteristics more similarly to the way they previously rated the friend's personality (means ± SE; for individual data points, see Figure S3A). This dynamic adjustment of self-concept to the “new” bodily self was enhanced for the participants who experienced strong illusory ownership of the friend's body, as indicated by the questionnaire (C) and skin conductance (D) measures.
Structural Reconfiguration of Self-Concept
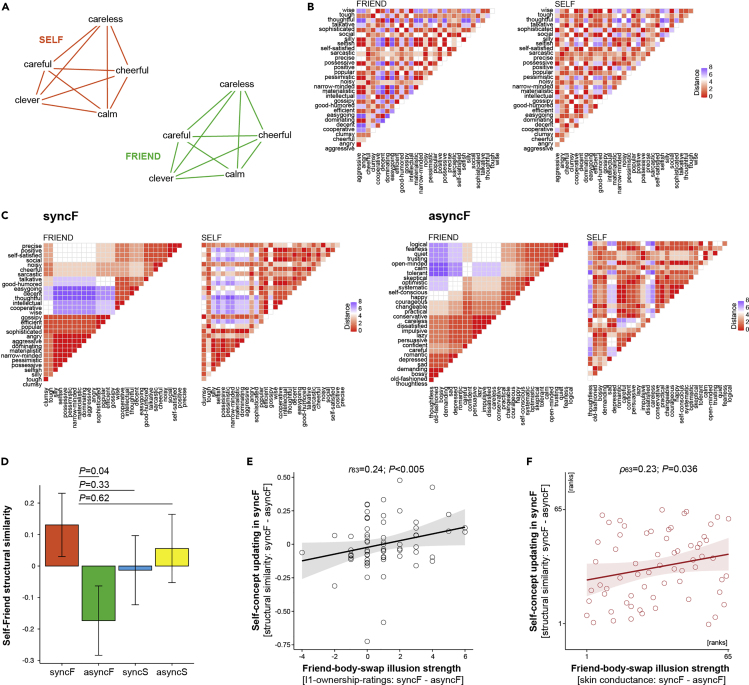
In a complementary post hoc approach, we next examined whether the illusion also reconfigured the multidimensional structure of self-concept and made it more similar to that of a friend-concept (Figure 4A). To this end, for each participant in each condition, we calculated the Euclidean distances between the ratings of every trait in relation to all other traits and compared how similar these distance matrixes were across beliefs about one's own and the friend's personalities in different conditions (Figure 4B). Please note that structural similarity as defined above is largely independent from the item-by-item similarity reported in the previous paragraph; for instance, a standard correlation between X [1 2 3 4 5] and Y [5 4 3 2 1] is −1, whereas the similarity of item relationships within X and Y is 1. We found that structural similarity between self-ratings and friend ratings was higher in the syncF than in the asyncF condition (Figures 4C and 4D; syncF vs. asyncF: b = 0.3; SE = 0.15; t = 2.05; p = 0.04; syncF vs. syncS: b = 0.14; SE = 0.15; t = 0.97; p = 0.33; syncF vs. asyncS: b = 0.07; SE = 0.15; t = 0.51; p = 0.62; LMMs; two-sided; N = 65). Furthermore, the stronger the friend-body-swap illusion, the higher the structural similarity between self-ratings and friend ratings in syncF; this significant relationship was present for the questionnaire and skin conductance measures of the illusion (Figures 4E and 4F, respectively; r63 = 0.24; p < 0.005; Pearson correlation; one-sided; ρ63 = 0.23; p = 0.036; Spearman correlations; one-sided; N = 65; see also Figure S4). It is noteworthy that the above analyses were performed on complex datasets, which makes it highly unlikely that the results were driven by the participants' conscious strategy or task compliance (see Limitations). Instead, the above findings provide complementary evidence that the moment-to-moment perception of one's own body reconfigures the multidimensional structure of beliefs about one's own personality.
Figure 4.
The Friend-Body-Swap Illusion Also Reconfigured the Multidimensional Structure of Self-Concept and Made It More Similar to the Structure of Friend-Concept
(A) A schematic illustration of “structural similarity.” Graphs represent ratings of five example personality traits provided with regard to the self (red) and the friend (green). Pairs of traits that are closer to each other were rated more similarly than traits that are farther apart. Structural similarity corresponds to resemblance between shapes of the whole graphs rather than between individual traits.
(B) For each participant in each condition, we calculated two distance matrixes: one between friend ratings (left) and the other between self-ratings (right). Both matrixes are based on the same traits (i.e., only the ones that were presented during a given condition). These unique “barcodes” of the participant's beliefs about one's own and friend's personalities correspond to distances between the ratings of each trait in relation to all other traits. For group analyses, we calculated a correlation between the self-matrix and the friend-matrix for each participant in each condition.
(C) Data from the syncF and asyncF conditions from a representative participant who experienced a strong friend-body-swap illusion (I1-ownership-ratings: syncF - asyncF = 4). For display purposes, trait adjectives are sorted according to the hierarchical clustering algorithm applied to friend ratings (“template”). Without sorting, the heatmaps in syncF would have looked like (B) (same data). Importantly, in the syncF condition, the main clusters of similar (low distance; red) and dissimilar (high distance; blue) ratings are largely preserved between the self- and friend-matrixes, resulting in high overall self-to-friend similarity (Spearman's rho = 0.54). In contrast, in the asyncF condition, the structure of clusters is very different between the self- and friend-matrixes, resulting in low overall self-to-friend similarity (Spearman's rho = 0.12).
(D) At the group level, structural similarity between self- and friend-ratings was higher in the syncF than in the asyncF condition (N = 65; means ± SE).
(E and F) The stronger the illusion of owning the friend's body—as measured by the illusion questionnaire ownership ratings (E) and threat-evoked skin conductance responses (F)—the greater the structural similarity between ratings of one's own and the friend's personalities in the syncF condition.
Self-Concept Updating and Episodic Recognition Memory
Moving to our second main hypothesis, we tested whether reinstating a coherent self-representation across the bodily and conceptual levels is beneficial for the ongoing encoding of episodic information. To this end, we asked the same participants to complete a memory task that was conducted immediately after the four full-body illusion conditions. During this task, the participants sat in front of computers (without experiencing any body-perception manipulation) and listened to the same trait adjectives as before that were now intermixed with 120 new trait characteristics (Data S1 and S2). The task was to indicate whether a given word had already occurred in the study (Figure 5A). Because the participants did not know about this memory test beforehand (i.e., memorizing traits was not explicitly required in the preceding personality rating part), it probed implicit (incidental) episodic recognition memory during the different full-body illusion conditions (Symons and Johnson, 1997). This paradigm is well suited for the current study because (1) it measures encoding of information in relation to the self-concept and (2) it complements the more explicit personality-ratings data. We found that memory performance for words encoded during the friend-body-swap illusion was generally reduced (Figure 5B; syncF vs. asyncF: b = −0.02; SE = 0.01; t = −3; p < 0.005; syncF vs. syncS: b = −0.02; SE = 0.01; t = −2.59; p = 0.013; syncF vs. asyncS: b = −0.02; SE = 0.01; t = −1.89; p = 0.063; LMMs; two-sided; N = 65). This was expected because the illusion of owning the friend's body should create a conflict between the bodily self and the self-concept and thus reduce overall self-coherence (Bergouignan et al., 2014; Bréchet et al., 2019). Importantly, however, high similarity between self-ratings and friend ratings during syncF was related to less impaired memory encoding in this condition (Figure 5C; ρ63 = −0.26; p = 0.029; Spearman correlation; two-sided). Analogously, among the participants who indicated high self-to-friend similarity during the friend-body-swap illusion, there was no significant difference between memory performance in syncF and other conditions (Figure 5D; memory: avg. (asyncF, syncS, asyncF) – syncF; t32 = 0.77; p = 0.44; N = 33; one-sample t test; two-sided). In contrast, the participants who showed weaker self-to-friend similarity during the illusion had significantly reduced recognition memory for traits encoded during the syncF condition (Figure 5D; memory: avg. (asyncF, syncS, asyncF) – syncF; t31 = 2.52; p < 0.005; N = 32; one-sample t test; two-sided). Control analyses indicated that these two subgroups of participants did not differ significantly with regard to potential confounding factors (Table S5). These results suggest that maintaining a coherent representation of ourselves across the bodily and conceptual levels is important for normal encoding of episodic information, whereas self-representation incoherence that is not compensated by self-concept updating impairs this encoding.
Figure 5.
Strong Updating of Self-Concept during the Friend-Body-Swap Illusion (syncF) Was Related to Less Impaired Recognition Memory of Personality Traits That Occurred during This Condition
(A) A schematic illustration of the memory task (orange, green, blue, and yellow bars represent items rated during syncF, asyncF, syncS, and asyncS, respectively; gray boxes represent new items).
(B) Memory performance for words encoded during the friend-body-swap illusion was generally reduced (means ± SE). For all subjects' individual data points, see Figure S3B.
(C) Strong updating of the self-concept toward the friend-concept during the friend-body-swap illusion was related to less impaired memory performance in syncF.
(D) Analogously, among the participants who showed strong updating of self-concept during syncF (≥median “similarity updating score”; syncF – avg. (asyncF, syncS, asyncS); N = 33), there was no significant difference between memory performance in syncF and in other conditions. In turn, the participants who showed weaker self-concept updating during syncF (<median “similarity updating score”; N = 32) remembered significantly fewer items from the syncF than from the control conditions (means ± SE). Please note that the median split was performed mainly for display purposes and to complement the main analyses shown in (B) and (C).
Memory Performance in asyncS
Finally, we tested whether memory encoding was also impaired by another type of self-incoherence, namely, by reduced ownership of one's own actual body in the asyncS condition (see syncS vs. asyncS; Figure 2). Indeed, we found that the participants who felt strong disownership of their real body in view during asyncS remembered fewer trait adjectives from this condition than from the syncS baseline condition (Figure 6; ρ63 = 0.28; p = 0.017; Spearman correlation; two-sided; N = 65). This result suggests that incoherence of the bodily self evoked by the disintegration of visual and somatosensory signals also impairs ongoing memory encoding.
Figure 6.
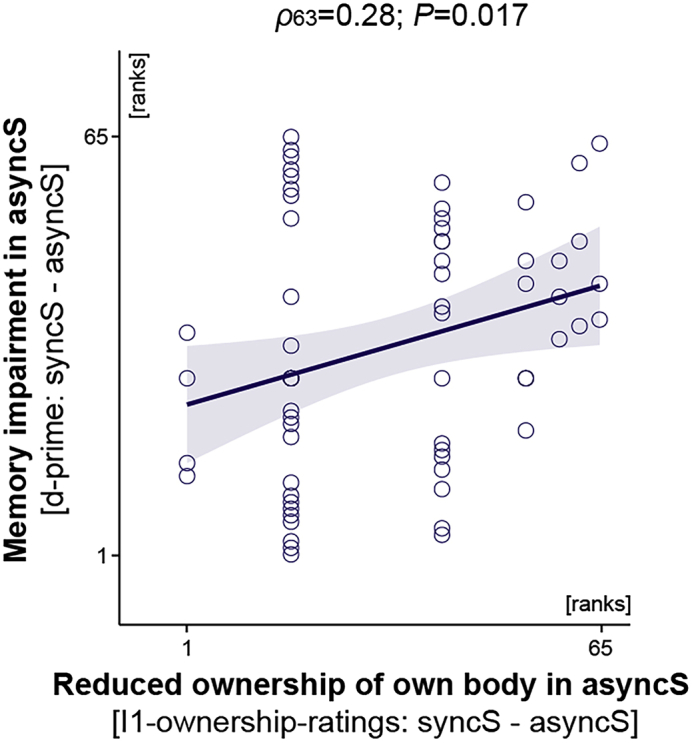
Reduced Ownership of One's Own Actual Body in asyncS Was Associated with More Impaired Recognition Memory of Traits That Were Rated during This Condition
Discussion
The present study examined the hypotheses that (1) the perception of one's own body (bodily self) dynamically shapes beliefs about one's own personality (self-concept) and (2) coherence between these two components of self-representation is important for normal memory encoding. With regard to the first hypothesis, we found that even a brief experience of illusory ownership of the friend's body changed the content and structure of multiple beliefs about one's own personality and made them more similar to beliefs about the friend's personality. This finding extends previous knowledge in several important ways. First, it challenges a common assumption that self-concept is relatively fixed over time and emphasizes the role of the body in the continuous construction of our sense of who we are; this role has been largely neglected in past social psychology research (Baumeister, 1998; Oyserman et al., 2012; Swann, 1997). Second, this result shows that perceptual aspects of the bodily self dynamically shape multiple, abstract beliefs that constitute our conscious self-concept rather than only selected aspects of self-representation that are perceptual, body-related, or implicit (Banakou et al., 2013; Sforza et al., 2010; Tajadura-Jimenez et al., 2012; Tsakiris, 2008; Yee et al., 2009). Third, this finding clarifies that the illusory ownership of another person's body not only modifies attitudes toward this person or toward a social group to which this person belongs (Maister et al., 2014, 2013; Paladino et al., 2010; Peck et al., 2013) but also, and perhaps predominantly, modifies beliefs about the self. Taken together, our results highlight the importance of the sense of one's own body as a foundation of social identity and self-concept.
With regard to possible cognitive mechanisms of this body-related updating of self-concept, embodied cognition theories propose that all concepts are grounded in sensorimotor representations (Barsalou, 2008); thus, a change in the representation of one's own body affects the content of self-concept. In turn, predictive processing theories suggest that, if the low-level perceptual representation of one's own body creates a conflict further up in the processing hierarchy, this conflict is resolved by updating higher-order beliefs about the self (Apps and Tsakiris, 2014; Tsakiris, 2017). Other studies have proposed that (1) illusory ownership of someone else's body involves making inferences about one's own attributes, e.g., “I am polite because the person whose body I have is polite” (Yee et al., 2009); (2) that the illusion allows new associations to be formed within the “self-image network” (Bedder et al., 2019); (3) that “owning” another person's body primes the concept of that person in the structure of knowledge (Peña et al., 2009); or (4) that body experiences of this kind increase the perceived physical similarity between the self and the other, which consequently increases the perceived conceptual similarity (Maister et al., 2014). What the present study adds to this complex discussion is the demonstration that self-concept updating is not a result of deliberate inference because the participants were not aware that their self-ratings became more similar to their friend ratings in the syncF condition (see further). Furthermore, the effect could not simply be explained by priming because the friend concept was likely “activated” even by looking at the friend's body during the asyncF condition. Instead, we found that the adjustment of self-concept toward the friend concept was tightly linked to the perceived strength of the illusory ownership of the friend's body (Figures 3C and 3D), which suggests that it was the multisensory experience of “having” the friend's body that drove the plastic changes of self-concept. As a novel hypothesis, we propose that (1) the brain represents self-concept as a convex region in the multidimensional space of different traits, skills, group-identities, etc. (Bellmund et al., 2018; Gärdenfors, 2004) and (2) because the illusion of having someone else's body greatly modifies the default way one experiences the self, this illusion leads to “remapping” of the self-concept in the above-mentioned multidimensional space. We speculate that at the neural level, this remapping is implemented by functional interactions between the multisensory fronto-parietal areas that represent perceptual aspects of the bodily self (Ehrsson et al., 2004; Petkova et al., 2011), the medial prefrontal region that is involved in the self-concept representation (Heatherton et al., 2006; Tacikowski et al., 2017), and the hippocampal-retrosplenial system that is related to spatial navigation, episodic memory, and translating between allocentric and egocentric mental perspectives (Andersen et al., 2007; Bergouignan et al., 2014; Burgess et al., 2001; Byrne et al., 2007; Eichenbaum, 2000; Guterstam et al., 2015b; Nyberg et al., 1996). Future neuroimaging and intracranial electrophysiology studies should investigate whether and, if so, how the multivariate representation of self-concept in the prefrontal cortex is “remapped” during illusory ownership of a familiar other's body.
Our second main finding is that the flexible adjustment of self-concept to the “new” bodily self is beneficial for the ongoing encoding of episodic information, whereas incoherence between the bodily and conceptual self-representations is associated with impaired memory processes. This finding is different from earlier experiments on out-of-body illusions (Bergouignan et al., 2014) and manipulations of the visual appearance of the body (Bréchet et al., 2019) that showed impaired encoding of episodic memories due to a disrupted sense of the bodily self (see further below). The present results establish that coherent self-representation across different levels—here, conceptual and bodily—plays an important role in normal mnemonic processing. Importantly, this finding cannot be explained by differences in the to-be-encoded material because traits in the present study were randomly assigned to different conditions. Furthermore, a context mismatch between the encoding and retrieval phases (Godden and Baddeley, 1975) cannot explain this finding because (1) memory performance in the most context-matching condition (syncS) was not higher than in all context-mismatching conditions (syncF, asyncF, asyncS) and (2) contextual cues related to visuotactile stimulation, the type of body in view, body position, etc., were all precisely controlled in our factorial experimental design. Finally, this finding cannot be explained by the possibility that the participants were just distracted during the friend-body-swap illusion and consequently missed some of the items in this condition because (1) all traits included in the memory dataset were associated with a button press in the preceding self-reference task; therefore, they were noticed and rated with similar task demands (i.e., the same instructions in all conditions) and (2) behavioral performance (i.e., reaction times and misses) did not differ significantly between conditions (see Transparent Methods). Instead, the present results suggest that coherence between different levels of self-representation is important for normal memory encoding, which advances our understanding of the fundamental relationship between the sense of self and memory. Regarding a possible mechanism, we speculate that a fragmented sense of self impairs the hippocampal binding mechanism during memory encoding (Andersen et al., 2007; Bergouignan et al., 2014; Eichenbaum, 2000; Nyberg et al., 1996) and makes it more difficult for this brain structure to integrate sensory and semantic information into coherent representations for long-term storage.
Two additional observations deserve brief discussion. First, visuotactile asynchrony in the asyncS condition reduced ownership of one's own actual body, which extends previous work that found such effects only for a single limb (Gentile et al., 2013; Kannape et al., 2019; Reader and Ehrsson, 2019) or when viewing oneself from a distance (Ehrsson, 2007; Guterstam et al., 2015b; Guterstam and Ehrsson, 2012). This result provides further support for multisensory models of full-body ownership (Ehrsson, 2020, 2012; Kilteni et al., 2015) by generalizing the temporal congruence principle—previously established in studies using mannequins (Petkova and Ehrsson, 2008), computer-simulated avatars (Slater et al., 2009), and unknown others (Preston and Ehrsson, 2014; Tacikowski et al., 2020)—to the case of one's real body viewed from a natural first-person perspective. Second, this reduced ownership of one's entire body was related to impaired memory performance, which goes beyond previous studies that found memory deficits (1) when the participants adopted a third-person perspective on their own body and changed self-location (Bergouignan et al., 2014) or (2) when the body was removed from view altogether (Bréchet et al., 2019). Evidently, a sense of reduced ownership of one's real body in view, even when it is observed from a first-person perspective, is sufficient to disrupt normal memory encoding. Interestingly, this disruption resembles the memory deficits of patients with depersonalization disorder who feel “detached” from their body (Giesbrecht et al., 2010; Sierra and David, 2011), which suggests that the current “full-body disownership illusion” could perhaps serve as an experimental model of memory impairments in this disorder. More generally, our memory findings during asyncS support the idea that reduced coherence of the multisensory representation of one's own body interrupts the hippocampal binding mechanisms engaged in episodic memory (Bergouignan et al., 2014).
In sum, we used a friend-body-swap illusion paradigm to show how self-concept is dynamically updated by changes in the bodily self and how the resulting increase in self-coherence facilitates memory encoding. These results advance our understanding of the basic link between the bodily self and self-concept as well as our understanding of the functional role of this connection. Moreover, the body-perception-induced fluidity of self-concept that we report here could have important implications for applied psychology; for example, it can be used as a therapeutic tool to promote more positive views of oneself in depression or to develop a deeper understanding between people by allowing them to literally experience the world from another person's perspective.
Limitations of the Study
A general concern with studies that use subjective ratings as a dependent variable, as we did, is how to rule out cognitive biases, task compliance, or suggestibility effects. In our opinion, such confounding factors cannot explain the current results for the following reasons. First, updating of the self-concept during syncF was related to increased skin-conductance responses, which are controlled largely automatically by the sympathetic nervous system (Dawson et al., 2000). Second, “producing” the structural similarity result would have been extremely difficult for the participants because it would have required them to maintain a mental overview of eight 30-by-30-item matrixes, be aware of the expected pattern of results, be able to quickly adjust their responses accordingly, and be able to simultaneously control their physiological reactions because the structural similarity finding was also related to enhanced skin-conductance responses. Third, memory results could not have been driven by cognitive bias because at the time of memory encoding, the participants did not know that a memory test would subsequently take place. Finally, we interviewed all the participants after the study, and none of the participants guessed the expected pattern of results (see Transparent Methods). Thus, even though illusory ownership of the friend's body updated the explicit content of people's self-concept, our findings suggest that this process of updating was largely implicit, which supports our main conclusions.
It is important to clarify that our memory task assessed episodic recognition memory, that is, whether the participants remembered encountering specific items during the preceding study session or not. Although this task is largely immune to the participants' possible conscious strategies (see earlier) and is relevant to the current research question (i.e., memory of items related to the self-concept is measured), task performance can be based on a feeling of familiarity even without a conscious recollection of how or when a particular item was encoded. Thus, we cannot determine whether the present findings can be generalized to other aspects of episodic memory, such as vividness or location at a specific time and place. Future studies should investigate this important question.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to the Lead Contact, Pawel Tacikowski (pawel.tacikowski@ki.se).
Materials Availability
All trait-stimuli are listed in the Data S1 and S2. For the illustration of the experimental setup, please see the Video S1. Captions are provided in the Supplemental Information file.
Data and Code Availability
Data underlying the study cannot be made publicly available owing to ethical concerns regarding participant privacy. We do not have ethics approval to make the raw data from individual subjects publicly available. The code is available from the Lead Contact on request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This study was funded by the Swedish Research Council (Distinguished Professor Grant 2017-03135 to H.H.E.), Torsten Söderbergs Stiftelse, Göran Gustafsons Stiftelse, and European Commission (MSCA fellowship awarded to P.T.; 750955). We would like to thank all the participants, Mattias Karlen for the illustration of the setup, and Martti Mercurio for important technical support.
Author Contributions
P.T. and H.H.E. designed the study. P.T. and M.L.W. collected and analyzed the data. P.T. and H.H.E. wrote the manuscript. All authors provided revisions and approved the final version of the manuscript for submission.
Declaration of Interests
The authors declare no competing interests.
Published: August 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101429.
Supplemental Information
The valence of each trait was indicated by independent raters (see Figure S6).
References
- Andersen P., Morris R., Amaral D., Bliss T., O’Keefe J. Oxford University Press; 2007. The hippocampus Book. [Google Scholar]
- Apps M.A.J., Tsakiris M. The free-energy self: a predictive coding account of self-recognition. Neurosci. Biobehav. Rev. 2014;41:85–97. doi: 10.1016/j.neubiorev.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspell J.E., Lenggenhager B., Blanke O. Keeping in touch with one’s self: multisensory mechanisms of self-consciousness. PLoS One. 2009;4:e6488. doi: 10.1371/journal.pone.0006488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banakou D., Groten R., Slater M. Illusory ownership of a virtual child body causes overestimation of object sizes and implicit attitude changes. Proc. Natl. Acad. Sci. U S A. 2013;110:12846–12851. doi: 10.1073/pnas.1306779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou L.W. Grounded cognition. Annu. Rev. Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Baumeister R.F. The self. In: Lindzey G., Gilbert D., Fiske S., editors. The Handbook of Social Psychology. Oxford University Press; 1998. pp. 680–740. [Google Scholar]
- Bedder R.L., Bush D., Banakou D., Peck T., Slater M., Burgess N. A mechanistic account of bodily resonance and implicit bias. Cognition. 2019;184:1–10. doi: 10.1016/j.cognition.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmund J.L.S., Gärdenfors P., Moser E.I., Doeller C.F. Navigating cognition: spatial codes for human thinking. Science. 2018;362:1–11. doi: 10.1126/science.aat6766. [DOI] [PubMed] [Google Scholar]
- Bergouignan L., Nyberg L., Ehrsson H.H. Out-of-body-induced hippocampal amnesia. Proc. Natl. Acad. Sci. U S A. 2014;111:4421–4426. doi: 10.1073/pnas.1318801111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O., Slater M., Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88:145–166. doi: 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Botvinick M., Cohen J. Rubber hands “feel” touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Bréchet L., Mange R., Herbelin B., Theillaud Q., Gauthier B., Serino A., Blankeid O. First-person view of one’s body in immersive virtual reality: influence on episodic memory. PLoS One. 2019;14:e0197763. doi: 10.1371/journal.pone.0197763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.D. Evaluations of self and others: self-enhancement biases in social judgements. Soc. Cogn. 1986;4:353–376. [Google Scholar]
- Brugger P., Lenggenhager B. The bodily self and its disorders: neurological, psychological and social aspects. Curr. Opin. Neurol. 2014;27:1–9. doi: 10.1097/WCO.0000000000000151. [DOI] [PubMed] [Google Scholar]
- Burgess N., Becker S., King J.A., O’Keefe J. Memory for events and their spatial context: models and experiments. Philos. Trans. R. Soc. B. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P., Becker S., Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.D., Trapnell P.D., Heine S.J., Katz I.M., Lavallee L.E., Lehman D.R. Self-concept clarity: measurement, personality correlates, and cultural boundaries. J. Pers. Soc. Psychol. 1996;70:141–156. [Google Scholar]
- Dawson M.E., Schell A.M., Filion D.L. The electrodermal system. In: Cacioppo J.T., Tassinary L., Berntson G., editors. Handbook of Psychophysiology. Cambridge University Press; 2000. pp. 200–223. [Google Scholar]
- Ehrsson H.H. Multisensory processes in body ownership. In: Sathian K., Ramachandran V., editors. Multisensory Perception: From Laboratory to Clinic. Academic Press; 2020. pp. 1–468. [Google Scholar]
- Ehrsson H.H. The concept of body ownership and its relation to multisensory integration. In: Stein B.E., editor. The New Handbook of Multisensory Processes. MIT Press; 2012. pp. 775–792. [Google Scholar]
- Ehrsson H.H. The experimental induction of out-of-body experiences. Science. 2007;317:1048. doi: 10.1126/science.1142175. [DOI] [PubMed] [Google Scholar]
- Ehrsson H.H., Spence C., Passingham R.E. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Festinger L. Stanford University Press; 1957. A Theory of Cognitive Dissonance. [Google Scholar]
- Gärdenfors P. Conceptual spaces as a framework for knowledge representation. Mind Matter. 2004;2:9–27. [Google Scholar]
- Gentile G., Guterstam A., Brozzoli C., Ehrsson H.H. Disintegration of multisensory signals from the real hand reduces default limb self-attribution: an fMRI study. J. Neurosci. 2013;33:13350–13366. doi: 10.1523/JNEUROSCI.1363-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht T., Merckelbach H., van Oorsouw K., Simeon D. Skin conductance and memory fragmentation after exposure to an emotional film clip in depersonalization disorder. Psychiatry Res. 2010;177:342–349. doi: 10.1016/j.psychres.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Godden D.R., Baddeley A.D. Context-dependent memory in two natural environments: on land and underwater. Br. J. Psychol. 1975;66:325–331. [Google Scholar]
- Guterstam A., Abdulkarim Z., Ehrsson H.H. Illusory ownership of an invisible body reduces autonomic and subjective social anxiety responses. Sci. Rep. 2015;5:9831. doi: 10.1038/srep09831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterstam A., Björnsdotter M., Gentile G., Ehrsson H. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr. Biol. 2015;25:1416–1425. doi: 10.1016/j.cub.2015.03.059. [DOI] [PubMed] [Google Scholar]
- Guterstam A., Ehrsson H.H. Disowning one’s seen real body during an out-of-body illusion. Conscious. Cogn. 2012;21:1037–1042. doi: 10.1016/j.concog.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Heatherton T.F., Wyland C.L., Macrae C.N., Demos K.E., Denny B.T., Kelley W.M. Medial prefrontal activity differentiates self from close others. Soc. Cogn. Affect. Neurosci. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J.B., Kang S.K. Mechanisms of identity conflict: uncertainty, anxiety, and the behavioral inhibition system. Personal. Soc. Psychol. Rev. 2016;20:223–244. doi: 10.1177/1088868315589475. [DOI] [PubMed] [Google Scholar]
- Kannape O.A., Smith E.J.T., Moseley P., Roy M.P., Lenggenhager B. Experimentally induced limb-disownership in mixed reality. Neuropsychologia. 2019;124:161–170. doi: 10.1016/j.neuropsychologia.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Kilteni K., Maselli A., Kording K.P., Slater M. Over my fake body: body ownership illusions for studying the multisensory basis of own-body perception. Front. Hum. Neurosci. 2015;9:141. doi: 10.3389/fnhum.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenggenhager B., Tadi T., Metzinger T., Blanke O. Video ergo sum: manipulating bodily self-consciousness. Science. 2007;317:1096–1099. doi: 10.1126/science.1143439. [DOI] [PubMed] [Google Scholar]
- Maister L., Sebanz N., Knoblich G., Tsakiris M. Experiencing ownership over a dark-skinned body reduces implicit racial bias. Cognition. 2013;128:170–178. doi: 10.1016/j.cognition.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maister L., Slater M., Sanchez-Vives M.V., Tsakiris M. Changing bodies changes minds: owning another body affects social cognition. Trends Cogn. Sci. 2014;19:6–12. doi: 10.1016/j.tics.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Nyberg L., Mcintosh A.R., Houle S., Nilsson L.-G., Tulving E. Activation of medial temporal structures during episodic memory retrieval. Nature. 1996;380:715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- Oyserman D., Elmore K., Smith G. Self, self-concept, and identity. In: Leary M.R., Tangney J.P., editors. Handbook of Self and Identity. The Guilford Press; 2012. pp. 69–104. [Google Scholar]
- Paladino M.-P., Mazzurega M., Pavani F., Schubert Synchronous multisensory stimulation blurs self-other boundaries. Psychol. Sci. 2010;21:1202–1207. doi: 10.1177/0956797610379234. [DOI] [PubMed] [Google Scholar]
- Peck T.C., Seinfeld S., Aglioti S.M., Slater M. Putting yourself in the skin of a black avatar reduces implicit racial bias. Conscious. Cogn. 2013;22:779–787. doi: 10.1016/j.concog.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Peña J., Hancock J.T., Merola N.A. The priming effects of avatars in virtual settings. Communic. Res. 2009;36:838–856. [Google Scholar]
- Petkova V.I., Bjornsdotter M., Gentile G., Jonsson T., Li T.Q., Ehrsson H.H. From part- to whole-body ownership in the multisensory brain. Curr. Biol. 2011;21:1118–1122. doi: 10.1016/j.cub.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Petkova V.I., Ehrsson H.H. If I were you: perceptual illusion of body swapping. PLoS One. 2008;3:e3832. doi: 10.1371/journal.pone.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postmes L., Sno H.N., Goedhart S., van der Stel J., Heering H.D., de Haan L. Schizophrenia as a self-disorder due to perceptual incoherence. Schizophr. Res. 2014;152:41–50. doi: 10.1016/j.schres.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Preston C., Ehrsson H.H. Illusory obesity triggers body dissatisfaction responses in the insula and anterior cingulate cortex. Cereb. Cortex. 2016;26:4450–4460. doi: 10.1093/cercor/bhw313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C., Ehrsson H.H. Illusory changes in body size modulate body satisfaction in a way that is related to non-clinical eating disorder psychopathology. PLoS One. 2014;9:e85773. doi: 10.1371/journal.pone.0085773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C., Kuper-Smith B.J., Ehrsson H.H. Owning the body in the mirror: the effect of visual perspective and mirror view on the full-body illusion. Sci. Rep. 2015;5:18345. doi: 10.1038/srep18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader A.T., Ehrsson H.H. Weakening the subjective sensation of own hand ownership does not interfere with rapid finger movements. PLoS One. 2019;14:e0223580. doi: 10.1371/journal.pone.0223580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.B., Kuiper N.A., Kirker W.S. Self-reference and the encoding of personal information. J. Pers. Soc. Psychol. 1977;35:677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Sforza A., Bufalari I., Haggard P., Aglioti S.M. My face in yours: visuo-tactile facial stimulation influences sense of identity. Soc. Neurosci. 2010;5:148–162. doi: 10.1080/17470910903205503. [DOI] [PubMed] [Google Scholar]
- Sierra M., David A.S. Depersonalization: a selective impairment of self-awareness. Conscious. Cogn. 2011;20:99–108. doi: 10.1016/j.concog.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Slater M., Perez-Marcos D., Ehrsson H.H., Sanchez-Vives M.V. Inducing illusory ownership of a virtual body. Front. Neurosci. 2009;3:214–220. doi: 10.3389/neuro.01.029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Humphreys G.W. The integrative self: how self-reference integrates perception and memory. Trends Cogn. Sci. 2015;19:719–728. doi: 10.1016/j.tics.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Swann W.B. The trouble with change: self-verification and allegiance to the self. Psychol. Sci. 1997;8:177–180. [Google Scholar]
- Symons C.S., Johnson B.T. The self-reference effect in memory: a meta-analysis. Psychol. Bull. 1997;121:371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Tacikowski P., Berger C.C., Ehrsson H.H. Dissociating the neural basis of conceptual self-awareness from perceptual awareness and unaware self-processing. Cereb. Cortex. 2017;27:3768–3781. doi: 10.1093/cercor/bhx004. [DOI] [PubMed] [Google Scholar]
- Tacikowski P., Fust J., Ehrsson H.H. 2020. Fluidity of Gender Identity Induced by Illusory Body-Sex Change. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajadura-Jimenez A., Grehl S., Tsakiris M. The other in me: interpersonal multisensory stimulation changes the mental representation of the self. PLoS One. 2012;7:e40682. doi: 10.1371/journal.pone.0040682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. The multisensory basis of the self: from body to identity to others. Q. J. Exp. Psychol. 2017;70:597–609. doi: 10.1080/17470218.2016.1181768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. Looking for myself: current multisensory input alters self-face recognition. PLoS One. 2008;3:e4040. doi: 10.1371/journal.pone.0004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M., Haggard P. The rubber hand illusion revisited: visuotactile integration and self-attribution. J. Exp. Psychol. Hum. Percept. Perform. 2005;31:80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- van der Hoort B., Guterstam A., Ehrsson H.H. Being Barbie: the size of one’s own body determines the perceived size of the world. PLoS One. 2011;6:e20195. doi: 10.1371/journal.pone.0020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee N., Bailenson J.N., Ducheneaut N. The Proteus effect implications of transformed digital self-representation on online and offline behavior. Communic. Res. 2009;36:285–312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The upper panels show what the participants are seeing in the Head Mounted Displays. For more information, please see the Results and Methods sections.
The valence of each trait was indicated by independent raters (see Figure S6).
Data Availability Statement
Data underlying the study cannot be made publicly available owing to ethical concerns regarding participant privacy. We do not have ethics approval to make the raw data from individual subjects publicly available. The code is available from the Lead Contact on request.