Abstract
Although Leishmania infantum is well-known as the aethiological agent of visceral leishmaniasis (VL), in some Central American countries it may cause atypical non-ulcerated cutaneous leishmaniasis (NUCL). However, the mechanisms favoring its establishment in the skin are still unknown. Lipophosphoglycan (LPG) is the major Leishmania multivirulence factor involved in parasite-host interaction. In the case of viscerotropic L. infantum, it causes an immunosuppression during the interaction with macrophages. Here, we investigated the biochemical and functional roles of LPGs from four dermotropic L. infantum strains from Honduras during in vitro interaction with murine macrophages. LPGs were extracted, purified and their repeat units analysed. They did not have side chains consisting of Gal(β1,4)Man(α1)-PO4 common to all LPGs. Peritoneal macrophages from BALB/c and C57BL/6 were exposed to LPG for nitric oxide (NO) and cytokine (TNF-α and, IL-6) production. LPGs from dermotropic strains from Honduras triggered higher NO and cytokine levels compared to those from viscerotropic strains. In conclusion, LPGs from dermotropic strains are devoid of side-chains and exhibit high pro-inflammatory activity.
Key words: lipophosphoglycan, Leishmania infantum, innate immunity
Leishmania infantum is well known as the cause of visceral leishmaniasis (VL) in the New World. In Central America, it has been shown that L. infantum also causes cutaneous lesions known as atypical cutaneous leishmaniasis (ACL) or non-ulcerated cutaneous leishmaniasis (NUCL). Early studies have already detected these forms in transmission areas in Costa Rica, El Salvador, Honduras and Nicaragua. In Honduras, parasitological examination of those strains from vertebrate and invertebrate hosts using isoenzymes allowed their identification as Leishmania donovani chagasi (nowadays L. infantum). 1 , 2 , 3 , 4 An interesting feature of NUCL is that in ACL patients no indication of prior visceralisation occurred and they were not immunocompromised and/or malnourished. 5 NUCL is a benign form affecting children and human immunodeficiency virus (HIV)-patients in Europe and their role as hosts in those endemic areas should be considered by public health authorities. 6 Most of the mechanisms underlying persistence of a viscerotropic species in the skin is still unknown. This is different from viscerotropic species L. donovani, where egested microbiota and IL-1β production are crucial for parasite migration from skin to organs. 7
In this context, several studies have assessed dermotropic L. infantum strains to understand their behavior in the skin. Macroscopically, NUCL is characterised by the presence of a small (0.1-3 cm) non-ulcerative erythematous papules surrounded by a hypopigmented halo on the exposed body areas including the face and extremities. 1 Recently, the immunopathological features of the lesions from Honduran strains were microscopically described. 8 , 9 Alike most dermotropic Leishmania species, 10 , 11 the pro-inflammatory infiltrated in the dermis consisted of mononuclear cells including lymphocytes, macrophages and a few plasma cells. An interesting feature of the lesions was the scarcity of parasites even if the infiltrates were discreet or intense. 8 Assessment of the local regulatory immune response in these lesions have detected the participation of FoxP3+ cells and TGF-β. 9 Altogether these studies suggest that a regulatory cellular immune response could promote low parasite persistence and tissue damage by ACL strains. Thus, these mechanisms could hinder parasite migration to organs as opposed to L. donovani IL-1β-induced inflammasome. 7 However, the role of parasite virulence factors in this process remains unknown.
Glycoconjugates of parasitic protozoans play a pivotal role during the parasite-host interaction. In Leishmania, lipophosphoglycan (LPG) is a multivirulence factor expressed on promastigote surface. LPG has four motifs: (i) a conserved glycan core region of 1-O-alkyl-2-lyso-phosphatidylnositol (PI); (ii) a core composed of Gal(α1,6)Gal(α1,3)Galf(β1,3)[Glc(α,1)-PO4]Man(α1,3)Man(α1,4)-GlcN(α1) heptasaccharide; (iii) a portion of phosphorylated repeat units Gal(β1,4) Man(α1)-PO4; and (iv), a terminal neutral oligosaccharide (cap). 12 Leishmania infantum LPG structures have been biochemically characterised in several strains from Brazil, Europe and Africa. Most of the strains possess type I LPG, whose repeat units are devoid of side chains. Only 10% of strains are branched-off with 1-3 β-glucose side-chains indicating the LPG polymorphisms are very low for L. infantum. 13 , 14 Several functions have been elucidated for L. infantum LPG during interaction with macrophages and other immune cells. These include: TLR2/TLR4 agonists, NF-kB translocation, induction of heme-oxygenase-1 and prostaglandin E2. 15 , 16 , 17 Compared to other dermotropic Leishmania species, L. infantum LPG exhibit a more immunosuppressive behavior, whereas L. braziliensis and L. amazonensis are pro-inflammatory. 15 , 18 Glycobiology studies on L. infantum has focused only on viscerotropic strains from humans and dogs and no information on LPG structures from dermotropic Central America strains is available.
As part of a wider study on L. infantum glycobiology, 13 , 14 this work purified and characterised the repeat units from dermotropic L. infantum causing NUCL in Honduras. Additionally, the activity of purified LPGs from dermotropic and viscerotropic was evaluated in murine macrophages.
All strains used in this study were from the Biorepository of Laboratorio de Patologias Infecciosas at University of São Paulo (USP). They were originally isolated from patients with NUCL from Amapala (Honduras) 8 , 9 and included: MHOM/HN/2017/AM-65, MHOM/HN/2017/AM-73, MHOM/HN/2018/AMA-161 and MHOM/HN/2018/AMA-614. Also, for functional macrophage studies, Brazilian viscerotropic strains were included (MHOM/BR/1970/BH46 and MCAN/BR/89/BA262). 14 Promastigotes were grown in M199 (Sigma, St. Louis, MO) and LPGs were extracted and purified from early stationary phase using a solvent E (H2O/ethanol/diethyl ether/pyridine/NH4OH; 15:15:5:1:0.017) (All from Merck, Darmstadt, Germany) as previously reported. 13 , 14 To confirm purification, LPGs were resolved in sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-rad, Berkeley, CA) and transferred to nitrocellulose paper (Bio-rad). The membrane was blocked for 1 h in 5% milk (Molico, Vevey, Vaud) in phosphate-buffered saline (PBS) and probed overnight with monoclonal antibody (mAb) CA7AE (1:1,000), which recognises the unsubstituted Gal(β1,4)Man repeat units. 19 After three washes in PBS, the membrane was incubated for 1 h with anti-mouse IgG conjugated with peroxidase (1:10,000) (Sigma) and the reaction was visualized using luminol (Bio-rad) (Fig. 1A). The LPGs from all dermotropic L. infantum strains were recognised by the mAb CA7AE, allowing the visualisation of characteristic smears common to all LPGs. 13 , 14 These results indicate that some repeat units were indeed unsubstituted. To confirm this, purified LPGs were subjected to mild acid hydrolysis (0.02 N HCl, 5 min, 100ºC) (Sigma) to depolymerize the repeat units. 13 Water-soluble fractions were partitioned using 1-butanol (Merck) and repeat units were treated with alkaline phosphatase (15 mM Tris buffer, pH 9,0, 1 U, 16 h, 37ºC) (Sigma). The neutral repeat units were desalted by passage through a two-layered column of AG50W-X12 (H+) over AG1-X8 (acetate) (Bio-rad). Then, samples were fluorescently labeled with 0.05 N ANTS (8-aminonaphthalene-1,3,6-trisulfate) and 1 M cyanoborohydride (37ºC, 16 h) (Sigma). They were subjected to fluorophore-assisted carbohydrate electrophoresis (FACE) using oligo-glucose ladders (G1-G7) 14 as standards (Sigma) (Fig. 1B). All strains exhibited only one band co-migrating with the standard oligo-glucose ladder Glc2 indicating the presence of the disaccharide Gal(β1,4) Man(α1). This profile is consistent with type I LPG, which was observed for 90% of the viscerotropic L. infantum strains. 14 Also, these LPGs were very similar to that from L. donovani (Sudan) and dermotropic species including L. braziliensis, Leishmania shawi and Leishmania enriettii. 20 , 21 , 22 , 23 Regardless of the tropism, LPGs from Old and New World L. infantum strains are devoid of side-chains reinforcing that this glycoconjugate has low polymorphism in this species. The qualitative information on LPG polymorphisms in the repeat units of L. infantum and L. donovani are summarised in Table.
Fig. 1: analysis of the lipophosphoglycans (LPGs) from dermotropic Leishmania infantum strains (AM-65, AM-73, AMA-161 and AMA-614): (A) Immunoblotting of purified intact LPG from promastigotes of L. infantum strains probed with mAb CA7AE (1:1,000). (B) Fluorophore-assisted carbohydrate electrophoresis (FACE) of LPG repeat units. Lane 1, oligo-glucose ladder represented by G1-G7; lanes 2-5, repeat units of AM-65, AM-73, AMA-161 and AMA-614 strains, respectively.
TABLE. Update on lipophosphoglycan (LPG) structures of Leishmania donovani complex species from Old and New World countries.
| Species/strainsa | Clinical patternb | Origin (city/statec /country) | LPG type | Ref. |
| Leishmania infantum | ||||
| MCAN/BR/89/Ba-262 | CanL | Jacobina/BA/Brazil | I | (14) |
| MHOM/BR/2001/HP-EMO | VL | Pancas/ES/Brazil | I | (14) |
| MHOM/BR/1987/HCO-1 | VL | ND/ES/Brazil | I | (14) |
| MCAN/BR/99/JP15 | CanL | João Pessoa/PB/Brazil | I | (14) |
| MHOM/BR/1985/GS | VL | ND/BA/Brazil | I | (14) |
| MHOM/BR/2003/MMF | VL | Cipolândia/MS/Brazil | I | (14) |
| 240 (dog/BR/ND) | CanL | Belo Horizonte/MG/Brazil | I | (14) |
| 291 (ND/BR/ND) | ND | Aracaju/SE/Brazil | I | (14) |
| MCAN/BR/2004/CUR268 | CanL | Belo Horizonte/MG/Brazil | I | (14) |
| MCAN/BR/2004/CUR269 | CanL | Belo Horizonte/MG/Brazil | I | (14) |
| MCAN/BR/2003/CUR211 | CanL | Belo Horizonte/MG/Brazil | I | (14) |
| MCAN/FR/1982/PHAROAH | CanL | ND/France | I | (14) |
| MHOM/TU/1980/IPT1 | VL | ND/Tunisia | I | (14) |
| MCAN/AL/1983/LIPA116 | CanL | ND/Algeria | I | (14) |
| MHOM/BR/74/PP75 | VL | Icatu/BA/Brazil | II | (13) |
| MHOM/BR/70/BH46 | VL | Conselheiro Pena/MG/Brazil | III | (14) |
| MHOM/HN/2017/AM-65 | NUCL | Amapala, Honduras | I | -- |
| MHOM/HN/2017/AM-73, | NUCL | Amapala, Honduras | I | -- |
| MHOM/HN/2018/AMA-161 | NUCL | Amapala, Honduras | I | -- |
| MHOM/HN/2018/AMA-614 | NUCL | Amapala, Honduras | I | -- |
| Leishmania donovani | ||||
| MHOM/SD/00/1S-2D | VL | ND/Sudan | I | (20) |
| MHOM/IN/1983/Mongi-142 | VL | ND/India | III | (27) |
a: The World Health Organization (WHO) code is as follows: host (MHOM, Homo sapiens; MCAN, Canis familiaris)/country/year of isolation/name of strain; b: VL = visceral leishmaniasis, CanL = canine leishmaniasis, NUCL = non-ulcerated cutaneous leishmaniasis, ND = not determined; c: Brazilian states (MG = Minas Gerais, BA = Bahia, PB = Paraíba, MS = Mato Grosso do Sul, ES = Espírito Santo, SE = Sergipe.
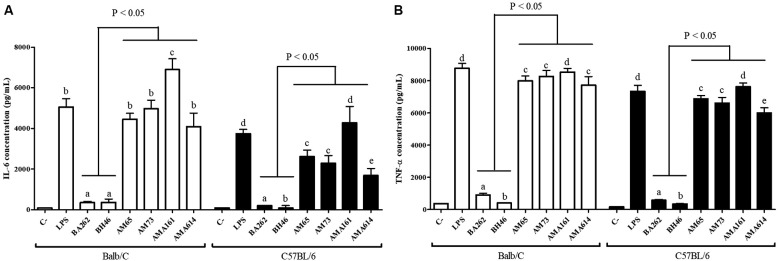
Next, we evaluated the pro-inflammatory activity of the LPGs from the four dermotropic strains of L. infantum (AM-65, AM-73, AMA-161 and AMA-614) compared to viscerotropic strains (BH46 and BA262). Thioglycollate-elicited (Sigma) peritoneal macrophages were removed from C57BL/6 and BALB/c mice by peritoneal washing. Cells (3.5 X 105 cells/well) were cultured in a sterile 96-well plate in Roswell Park Memorial Institute (RPMI) medium (Gibco, Waltham, MA) and primed with IFN-γ (100 IU/mL) (R&D Systems, Minneapolis, MN). 18 Macrophages were exposed to lipopolysaccharide (LPS) (Sigma) (0.1 µg/mL - positive control); LPGs (10 µg/mL) and RPMI 1640 medium only (negative control). Culture supernatants were collected after 72 h and nitrite concentrations were determined by Griess reaction (Sigma). IL-6 and TNF-α were determined using BD CBA Mouse Cytokine assay kits according to the manufacturer’s specifications (BD Biosciences, CA, USA). 18 In general, LPGs from all dermotropic strains were more pro-inflammatory than those from viscerotropic strains not only in BALB/c but also in C57BL/6 mice. In both mouse lineages, LPGs from dermotropic strains were comparable to LPS (positive control) or even higher (strain AMA-161) in their ability to induce NO, IL-6 and TNF-α (Fig. 2, Fig. 3A-B). Additionally, inner intraspecies variations were observed in the viscerotropic strains LPGs for both mice (BH46 versus BA262, p < 0.05). The low ability of BA262 LPG in inducing NO synthesis was already reported. 14 Consistent with these observations, LPG1 knockouts of this strain induced higher levels of NO in RAW 264.7 cells confirming the role of LPG in this process. 24 For dermotropic strains these differences were more evident for BALB/c (AMA-161 versus AM-65, AM-73 and AMA-614, p < 0.05). In C57BL/6, a higher NO production was detected for AMA-161, followed by AM-65 and AMA-73/AMA-614 (p < 0.05). Previous reports from our group 14 showed that differences in LPG structures in L. infantum were determinant for NO production. This correlation was not easily demonstrated here, suggesting that perhaps intraspecies polymorphisms in the lipid anchors and/or the length of the repeat units motif of the dermotropic strains could also be responsible for higher NO and cytokine induction. 25 , 26 Depending on the species and/or glycoconjugates, NO production was usually higher for C57BL/6 mice and did not show major variations for cytokines. 14 , 26 However, these studies used a limited number of strains. Here, with an expanded panel of L. infantum strains, we decided to use both mice subsets for comparison. Interestingly, NO, IL-6 and TNF-α production were similar for both mice lineages. LPG from strain AMA-161 was observed even in levels higher than those of LPS (Figs 2 and 3, p < 0.05). Confirming our previous observations, 15 NO and cytokine induction by BH46 LPG was very low in macrophages from BALB/c and C57BL/6 mice. Overall, macrophages activation by LPG from dermotropic species was much higher than those from the two viscerotropic strains (BA262 and BH46) (Figs 2 and 3, p < 0.05). These strains possess type I and type III LPGs, respectively. Since the LPGs from all Honduran strains are type I. As mentioned above, differences in stimulation could be not only a result of the length of the LPG but also the type of lipid anchor. LPG has four parts and several reports have shown that both glycan and lipid motifs are important for macrophage stimulation not only by LPGs but also for glycoinositolphospholipids (GIPLs). 25 , 26 In vitro experiments with dermotropic strains did not show evident differences in their ability to infect and survive in hamster peritoneal macrophages (MD Laurenti, Unpublished observations). This strain was isolated from an older patient (69 years), whose lesion had a longer evolution time (3 years), which could be reflecting a more balanced parasite-host adaptation. This higher NO stimulation by LPGs from dermotropic species, together with TGF-β, may explain low parasite loads observed in those lesions. 9 This was very surprising since LPGs from viscerotropic species (L. infantum and L. donovani are usually more immunosuppressive. 15 , 27 , 28 , 29 This suggests that the activity of LPGs from dermotropic L. infantum resembles to that of L. braziliensis, L. enriettii and L. amazonensis. 15 , 18 , 23 , 30 This reinforces that dermotropic L. infantum with respect to these mediators (NO and cytokines) are behaving very similar to cutaneous species.
Fig. 2: nitric oxide (NO) production by murine peritoneal macrophages (BALB⁄c and C57BL/6) exposed to lipophosphoglycan (LPG) (10 µg/mL) from dermotropic (AM65, AM73, AMA161 and AMA614) and viscerotropic strains (BA262 and BH46) of Leishmania infantum. Lipopolysaccharide (LPS) (0.1 µg/mL) was used as positive control. Results were expressed as the mean ± standard deviation of three independent experiments. Statistical analysis was achieved using the nonparametric Kruskal-Wallis test followed by Dunn’s post hoc test for multiple comparisons among groups (lines above bars). T-Student´s t test was used to compare each sample and letters above bars indicate statistical differences (p < 0.05).
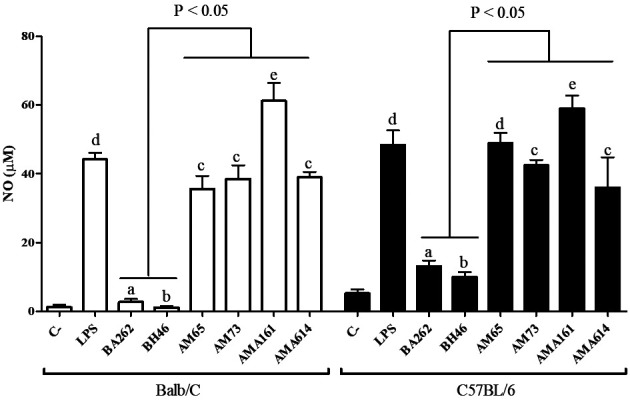
Fig. 3: IL-6 (A) and TNF-α (B) production by murine peritoneal macrophages (BALB/c and C57BL/6) exposed to lipophosphoglycan (LPG) (10 µg/mL) from dermotropic (AM65, AM73, AMA161 and AMA614) and viscerotropic strains (BA262 and BH46) of Leishmania infantum. Lipopolysaccharide (LPS) (0.1 µg/mL) was used as positive control. Results were expressed as the mean ± standard deviation of three independent experiments. Statistical analysis was achieved using the nonparametric Kruskal-Wallis test followed by Dunn’s post hoc test for multiple comparisons among groups (lines above bars). T-Student´s t test was used to compare each sample and letters above bars indicate statistical differences (p < 0.05).
In conclusion, L. infantum has been shown to cause a wide spectrum of manifestations, from benign skin lesions to fatal visceral forms. In this work, we characterised the structure of the LPG from four NUCL strains. Despite the LPGs having a type I structure, they triggered higher NO and cytokine production than those from viscerotropic strains, a pattern often observed in other cutaneous Leishmania species.
Ethics statement - All animals were handled in strict accordance with animal practice as defined by the Internal Ethics Committee in Animal Experimentation (CEUA) of Fundação Oswaldo Cruz (FIOCRUZ), Belo Horizonte, Minas Gerais (MG), Brazil (protocol P-17/14-2). This protocol followed the guidelines of CONCEA/MCT.
ACKNOWLEDGEMENTS
To Jason Memmott for English review.
Footnotes
Financial support: CNPq, FAPEMIG, FAPESP. RPS, CAC, PMN and MDL are supported by CNPq (127095/2019-5, 167727/2017-6, 302174/2017-6 and 302972/2019-6), FAPEMIG (PPM-XII 00202-18) and FAPESP (2014/50315-0, 2017/24834-9 and 2018/04698-6). GVA and CMS are supported by FAPESP. WHS is supported by Dirección de Investigacion y Posgrados de la UNAH (grant #02-2015).
REFERENCES
- 1.Ponce C, Ponce E, Morrison A, Cruz A, Kreutzer R, McMahon-Pratt D. Leishmania donovani chagasi new clinical variant of cutaneous leishmaniasis in Honduras. Lancet. 1991;337(8733):67–70. doi: 10.1016/0140-6736(91)90734-7. [DOI] [PubMed] [Google Scholar]
- 2.Noyes H, Hance M, Ponce C, Ponce E, Maingon R. Leishmania chagasi genotypically similar parasites from Honduras cause both visceral and cutaneous leishmaniasis in humans. Exp Parasitol. 1997;85(3):264–273. doi: 10.1006/expr.1996.4133. [DOI] [PubMed] [Google Scholar]
- 3.Zeledón R, Hidalgo H, Víquez A, Urbina A. Atypical cutaneous leishmaniasis in a semiarid region of north-west Costa Rica. Trans R Soc Trop Med Hyg. 1989;83(6):786–786. doi: 10.1016/0035-9203(89)90328-3. [DOI] [PubMed] [Google Scholar]
- 4.Belli A, Marín F, Valle S, Palacios X, Videa E, García D. Widespread atypical cutaneous leishmaniasis caused by Leishmania (L ) chagasi in Nicaragua. Am J Trop Med Hyg. 1999;61(3):380–385. doi: 10.4269/ajtmh.1999.61.380. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Ponce M, Ponce C, Ponce E, Maingon RDC. Leishmania chagasi/infantum further investigations on Leishmania tropisms in atypical cutaneous and visceral leishmaniasis foci in Central America. Exp Parasitol. 2005;109(4):209–219. doi: 10.1016/j.exppara.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Cunha J, Carrillo E, Sánchez C, Cruz I, Moreno J, Cordeiro-da-Silva A. Characterization of the biology and infectivity of Leishmania infantum viscerotropic and dermotropic strains isolated from HIV+ and HIV-patients in the murine model of visceral leishmaniasis. Parasit Vectors. 2013;6:122–122. doi: 10.1186/1756-3305-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey R, Joshi AB, Oliveira F, Pereira L, Guimarães-Costa AB, Serafim TD. Gut microbes egested during bites of infected sand flies augment severity of leishmaniasis via inflammasome-derived IL-1ß Cell Host. Microbe. 2018;23(1):134–143. doi: 10.1016/j.chom.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacheco CMS, Flores GVA, Ferreira AF, Ochoa WS, da Matta VLR, Valeriano CZ. Histopathological features of skin lesions in patients affected by non-ulcerated or atypical cutaneous leishmaniasis in Honduras, Central America. Int J Exp Pathol. 2018;99(5):249–257. doi: 10.1111/iep.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores GVA, Pacheco CMS, Tomokane TY, Ochoa WS, Valeriano CZ, Gomes CMC. Evaluation of regulatory immune response in skin lesions of patients affected by nonulcerated or atypical cutaneous leishmaniasis in Honduras, Central America. Mediators Inflamm. 2018;2018:3487591–3487591. doi: 10.1155/2018/3487591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinheiro LJ, Paranaiba LF, Alves A, Parreiras PM, Gontijo NF, Soares RP. Salivary gland extract modulates the infection of two Leishmania enriettii strains by interfering with macrophage differentiation in the model of Cavia porcellus. Front Microbiol. 2018;9:969–969. doi: 10.3389/fmicb.2018.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marques FAG, Soares RP, Almeida GG, Souza CC, Melo MN, Pinto SA. Effectiveness of an immunohistochemical protocol for Leishmania detection in different clinical forms of American tegumentary leishmaniasis. Parasitol Int. 2017;66(2017):884–888. doi: 10.1016/j.parint.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 12.de Assis RR, Ibraim IC, Nogueira PM, Soares RP, Turco SJ. Glycoconjugates in New World species of Leishmania polymorphisms in lipophosphoglycan and glycoinositolphospholipids and interaction with hosts. Biochim Biophys Acta (BBA)-General Subjects. 2012;1820(9):1354–1365. doi: 10.1016/j.bbagen.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Soares RP, Macedo ME, Ropert C, Gontijo NF, Almeida IC, Gazzinelli RT. Leishmania chagasi lipophosphoglycan characterization and binding to the midgut of the sand fly vector Lutzomyia longipalpis. Mol Biochem Parasitol. 2002;121(2002):213–224. doi: 10.1016/s0166-6851(02)00033-6. [DOI] [PubMed] [Google Scholar]
- 14.Coelho-Finamore JM, Freitas VC, Assis RR, Melo MN, Novozhilova N, Secundino NF. Leishmania infantum lipophosphoglycan intraspecific variation and interaction with vertebrate and invertebrate hosts. Int J Parasitol. 2011;41(2011):333–342. doi: 10.1016/j.ijpara.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Ibraim IC, Assis RR, Pessoa NL, Campos MAS, Melo MN, Turco SJ. Two biochemically distinct lipophosphoglycans from Leishmania braziliensis and Leishmania infantum trigger different innate immune responses in murine macrophages. Parasit Vectors. 2013;6:54–54. doi: 10.1186/1756-3305-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luz NF, Andrade BB, Feijó DF, Araújo-Santos T, Soares RP, Quintela GC. Heme oxygenase-1 promotes the persistence of Leishmania chagasi infection. J Immunol. 2012;188(9):4460–4467. doi: 10.4049/jimmunol.1103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima JB, Araújo-Santos T, Souza ML, Brito A, Luz NF, Jesus-Santos FH. Leishmania infantum lipophosphoglycan-induced Prostaglandin E2 production in association with PPAR- expression via activation of Toll like receptors-1 and 2. Sci Rep. 2017;7(1):14321–14321. doi: 10.1038/s41598-017-14229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueira PM, Assis RR, Melo MN, Saraiva EM, Pessoa NL, Campos MAS. Lipophosphoglycans from Leishmania amazonensis strains display immunomodulatory properties via TLR4 and do not affect sand fly infection. PLoS Negl Trop Dis. 2016;10(8):e0004848. doi: 10.1371/journal.pntd.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolson DL, Turco SJ, Beecroft RP, Pearson TW. The immunochemical structure and surface arrangement of Leishmania donovani lipophosphoglycan determined using monoclonal antibodies. Mol Biochem Parasitol. 1989;35(2):109–118. doi: 10.1016/0166-6851(89)90113-8. [DOI] [PubMed] [Google Scholar]
- 20.Sacks DL, Pimenta PFP, McConville MJ, Schneider P, Turco SJ. Stage-specific binding of Leishmania donovani to the sand fly vector midgut is regulated by conformational changes in the abundant surface lipophosphoglycan. J Exp Med. 1995;181(2):685–697. doi: 10.1084/jem.181.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soares RP, Cardoso TL, Barron T, Araujo MS, Pimenta PF, Turco SJ. Leishmania braziliensis a novel mechanism in the lipophosphoglycan regulation during metacyclogenesis. Int J Parasitol. 2005;35(2005):245–253. doi: 10.1016/j.ijpara.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Passero FLD, Assis RR, Nogueira PM, Macedo DH, Campos MAS, Pessoa NL. Differential pro-inflammatory activity between glycoinositolphospholipids and lipophosphoglycan from Leishmania shawi. Parasitol Int. 2015;64(2015):32–35. doi: 10.1016/j.parint.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Paranaíba LF, Assis RR, Nogueira PM, Torrecilhas AC, Silveira ACO, Martins-Filho AO. Leishmania enriettii biochemical characterisation of lipophosphoglycans (LPGs) and glycoinositolphospholipids (GIPLs) and infectivity to Cavia porcellus. Parasit Vectors. 2015;8:31–31. doi: 10.1186/s13071-015-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lázaro-Souza M, Matte C, Lima JB, Duque GA, Quintela-Carvalho G, Vivarini AC. Leishmania infantum lipophosphoglycan-deficient mutants a tool to study host cell-parasite interplay. Front Microbiol. 2018;9:626–626. [Google Scholar]
- 25.de Veer MJ, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, McConville MJ. MyD88 is essential for clearance of Leishmania major possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol. 2003;33(10):2822–2831. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 26.Assis RR, Ibraim IC, Noronha FS, Turco SJ, Soares RP. Glycoinositolphospholipids from Leishmania braziliensis and L infantum: modulation of innate immune system and variations in carbohydrate structure. PLoS Negl Trop Dis. 2012;6(2):e1543. doi: 10.1371/journal.pntd.0001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahoney AB, Sacks DL, Saraiva E, Modi G, Turco SJ. Intra-species and stage-specific polymorphisms in lipophosphoglycan structure control Leishmania donovani-sand fly interactions. Biochemistry. 1999;38(31):9813–9823. doi: 10.1021/bi990741g. [DOI] [PubMed] [Google Scholar]
- 28.McNeely TB, Turco SJ. Inhibition of protein kinase C activity by the Leishmania donovani lipophosphoglycan. Biochem Biophys Res Commun. 1987;148(1987):653–657. doi: 10.1016/0006-291x(87)90926-0. [DOI] [PubMed] [Google Scholar]
- 29.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163(12):6403–6412. [PubMed] [Google Scholar]
- 30.Vieira TDS, Rugani JN, Nogueira PM, Torrecilhas AC, Gontijo CMF, Descoteaux A. Intraspecies polymorphisms in the lipophosphoglycan of L braziliensis differentially modulate macrophage activation via TLR4. Front Cell Infect Microbiol. 2019;9:240–240. doi: 10.3389/fcimb.2019.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]