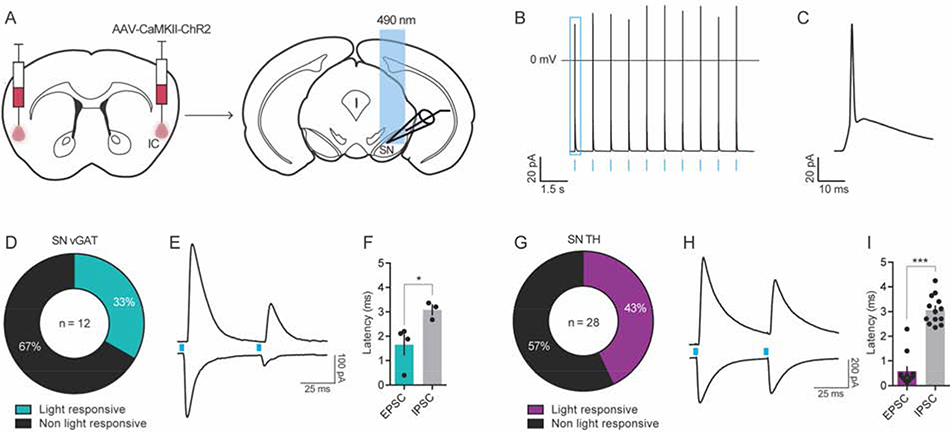
Figure 8. The IC projects polysynaptically onto GABA and dopamine neurons of the SN.
(A) Channelrhodopsin-2 (ChR2)-assisted circuit mapping was used to identify the cell populations in the SN innervated by IC inputs. (B) Functional expression of ChR2 was confirmed using patch clamp electrophysiology, which showed that IC ChR2+ neurons fired action potentials in response to optogenetic cell body stimulation (blue bars) at 1 Hz. (C) Zoomed in example of a light-evoked action potential from panel B (in blue square). (D-I) Optically-evoked excitatory (oEPSC) and inhibitory postsynaptic current (oIPSC) were recorded in SN vGAT (D-F) and TH (G-I) neurons after ChR2 stimulation. (D) 33% of vGAT neurons were light responsive, with n = 4 / 12 showing a time-locked (< 2 ms latency) oEPSC and n = 3 / 12 cells showing a delayed (> 3 ms latency) polysynaptic oIPSC to a 5 ms blue-light pulse (F). (G-I) In TH neurons, 43% were light-responsive, with n = 12 / 28 showing a time-locked (< 1 ms latency) oEPSC and n = 13 / 28 showing a delayed (> 3 ms latency) polysynaptic oIPSC (I). Representative traces of light-evoked oEPSC (bottom) and oIPSC (top) in vGAT neurons (E) and TH neurons (H) in the SN during the paired pulse stimulation protocol, where blue boxes indicated light pulse stimulus onset. Latency of oIPSC onset following light stimulation was significantly greater than that of oEPSC illustrating the polysynaptic nature of this input to SN vGAT (F) and TH neurons (I), *p < 0.05, ***p < 0.001; TH, tyrosine hydroxylase; vGAT, vesicular GABA transporter