Abstract
Background
Obesity is common in patients with coronavirus disease 2019 (COVID-19). The effects of obesity on clinical outcomes of COVID-19 warrant systematical investigation.
Objective
This study explores the effects of obesity with the risk of severe disease among patients with COVID-19.
Methods
Body mass index (BMI) and degree of visceral adipose tissue (VAT) accumulation were used as indicators for obesity status. Publication databases including preprints were searched up to August 10, 2020. Clinical outcomes of severe COVID-19 included hospitalization, a requirement for treatment in an intensive care unit (ICU), invasive mechanical ventilation (IMV), and mortality. Risks for severe COVID-19 outcomes are presented as odds ratios (OR) and 95% confidence interval (95%CI) for cohort studies with BMI-defined obesity, and standardized mean difference (SMD) and 95%CI for controlled studies with VAT-defined excessive adiposity.
Results
A total of 45, 650 participants from 30 studies with BMI-defined obesity and 3 controlled studies with VAT-defined adiposity were included for assessing the risk of severe COVID-19. Univariate analyses showed significantly higher ORs of severe COVID-19 with higher BMI: 1.76 (95%: 1.21, 2.56, P = 0.003) for hospitalization, 1.67 (95%CI: 1.26, 2.21, P<0.001) for ICU admission, 2.19 (95%CI: 1.56, 3.07, P<0.001) for IMV requirement, and 1.37 (95%CI: 1.06, 1.75, P = 0.014) for death, giving an overall OR for severe COVID-19 of 1.67 (95%CI: 1.43, 1.96; P<0.001). Multivariate analyses revealed increased ORs of severe COVID-19 associated with higher BMI: 2.36 (95%CI: 1.37, 4.07, P = 0.002) for hospitalization, 2.32 (95%CI: 1.38, 3.90, P = 0.001) for requiring ICU admission, 2.63 (95%CI: 1.32, 5.25, P = 0.006) for IMV support, and 1.49 (95%CI: 1.20, 1.85, P<0.001) for mortality, giving an overall OR for severe COVID-19 of 2.09 (95%CI: 1.67, 2.62; P<0.001). Compared to non-severe COVID-19 patients, severe COVID-19 cases showed significantly higher VAT accumulation with a SMD of 0.49 for hospitalization (95% CI: 0.11, 0.87; P = 0.011), 0.57 (95% CI: 0.33, 0.81; P<0.001) for requiring ICU admission and 0.37 (95% CI: 0.03, 0.71; P = 0.035) for IMV support. The overall SMD for severe COVID-19 was 0.50 (95% CI: 0.33, 0.68; P<0.001).
Conclusions
Obesity increases risk for hospitalization, ICU admission, IMV requirement and death among patients with COVID-19. Further, excessive visceral adiposity appears to be associated with severe COVID-19 outcomes. These findings emphasize the need for effective actions by individuals, the public and governments to increase awareness of the risks resulting from obesity and how these are heightened in the current global pandemic.
Abbreviations: COVID-19, Coronavirus Disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ICU, intensive care unit; BMI, body mass index; CNKI, Chinese National Knowledge Infrastructure; MeSH, Medical Subject Headings; VAT, Visceral adipose tissue; IMV, invasive mechanical ventilation; OR, odds ratio; 95%CI, 95% confidence interval; SMD, standardized mean difference; NOS, Newcastle-Ottawa Scale; CT, computed tomography; IAV, Influenza A virus; ACE2, angiotensin-converting enzyme 2; SAT, subcutaneous adipose tissue; DKD, diabetic kidney disease
Keywords: Obesity, Coronavirus disease 2019, Visceral adipose tissue, Intensive care, Invasive mechanical ventilation, Mortality
Highlights
-
•
Obesity increases risk for hospitalization among patients with COVID-19.
-
•
Obesity increases risk for needing ICU admission among patients with COVID-19.
-
•
Obesity increases risk for requiring IMV support among patients with COVID-19.
-
•
Obesity increases risk for death among patients with COVID-19.
-
•
Excessive visceral adiposity appears to be associated with severe COVID-19 outcomes.
1. Introduction
The world is witnessing a global pandemic of coronavirus disease 2019 (COVID-19), which is considered to be related to infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Most people with COVID-19 appear to develop mild to moderate illness characterized by fever, dry cough and tiredness, and recover without intensive care unit (ICU) admission [1]. In some individuals, however, it may progress to serious conditions such as pneumonia and respiratory failure. As of 10 August 2020, there were 19, 718, 030 confirmed cases and 728, 013 confirmed deaths reported in 216 countries, areas or territories [2]. As the crisis of COVID-19 continues, worldwide efforts have been underway to investigate the disease severity and health complications. Early studies have shed light on risk factors that might drive the disease to be life threatening [3,4]. Patients who are older and have pre-existing chronic medical conditions, including obesity, cardiovascular diseases, diabetes, cancers and chronic respiratory diseases and kidney diseases were found to be vulnerable to severe COVID-19 [3,4].
Of concern, most chronic medical conditions often co-exist with obesity even in patients younger than 60 years of age. As a public health epidemic, obesity affects more than 650 million adults (about 13% of the world's adult population) and 124 million children and adolescents worldwide [5]. Moreover, much clinical research from different affected countries and areas suggests a strong relationship between body mass index (BMI) defined obesity and increased risk of testing positive for SARS-CoV-2 [6], as well as increased risk of severe disease among patients with COVID-19 [[7], [8], [9], [10]]. In a prospective cohort study of 233 patients hospitalized with COVID-19 in Italy, patients with obesity had a 3-fold higher risk of death as compared to those with a BMI<30 kg/m2 [11]. Among 200 patients with COVID-19 in the Bronx borough of New York City, severe obesity (defined as BMI ≥ 35 kg/m2) was associated with higher in-hospital mortality independent of other pertinent potentially confounding factors [12]. However, a prospective cohort study of 1150 adults hospitalized with COVID-19 in New York did not show that severe obesity was an independent risk factor for in-hospital mortality [13]. Other preliminary studies have suggested that increased visceral fat is associated with a worse prognosis among patients with COVID-19 [14,15], implicating a potential positive association between visceral obesity and COVID-19 severity. Therefore, the effects of obesity on the clinical outcomes of COVID-19 warrant systematical investigation. We conducted this systematic review and meta-analysis to investigate the impact of obesity on disease severity and fatality among patients with COVID-19.
2. Material and methods
2.1. Literature search
Medical articles published or preprinted up to August 10, 2020 were searched through databases including PubMed, EMBASE, Web of Science, Chinese National Knowledge Infrastructure (CNKI), Wanfang Data, SinoMed, and the preprint service for the medicine and health sciences of medRxiv, with no restriction on the language used. We searched databases of published articles with text-words in Medical Subject Headings (MeSH) and the text-words are as follows: (“coronavirus disease 2019” or “covid-19” or “2019 novel coronavirus” or “2019-ncov” or “novel coronavirus 2019 infection” or “2019-ncov infection” or “severe acute respiratory syndrome coronavirus 2” or “sars-cov-2”) AND “obesity” or “overweight” or “body mass index” or “BMI” or “visceral fat” or “excessive fat” or “abdominal fat” or “visceral adipose tissue” or “visceral adiposity” or “central adiposity” or “waist circumference” or “risk factors” or “factor” or “risk factor” or “clinical characteristics” or “clinical features”. For medRxiv searching, we used the term of “covid-19 and obesity” as the strategy to identify the potentially most relevant articles for our study.
BMI and VAT levels were used as indicators to reflect obesity status in the present analysis. The amount of VAT was measured by using computed tomography (CT), and all defined-indicators of quantification of VAT among studies were accepted. Severe COVID-19 was defined by four clinical outcomes including three levels of care that a patient required, and the worst outcome of death. The three levels of care were hospitalization, ICU admission, and invasive mechanical ventilation (IMV) support. Death related to COVID-19 was defined as being a lab-confirmed case of COVID-19 death during the study period and the follow-up period of a study, regardless of where or not the death occurred in a hospital. Diagnostic criteria for cases of COVID-19 in studies required a laboratory-confirmed positive SARS-CoV-2 infection as a necessary condition. All diagnostic criteria for obesity among studies were accepted. Based on these criteria, we searched for cohort studies on COVID-19 among published articles and other publicly available research that met the following eligibility criteria: (a) BMI or VAT amount or obesity status provided in the data of demographic characteristics; and (b) Reported clinical outcomes included hospitalization, ICU admission, IMV requirement, and death for study participants with COVID-19. We excluded medical literatures with the following characteristics: (a) participants in the control group for cohort study were people without COVID-19; (b) pregnant women with COVID-19 were the object of study; (c) participants of a study included suspected cases of COVID-19 which were not confirmed by SARS-CoV-2 diagnostic tests. (d) no defined obesity group data or BMI data were provided as continuous variables rather than categorical that indicated obesity status; (e) no available data were provided for the calculation of odds ratio (OR) and 95% confidence interval (95%CI) when indicating the strength of association between BMI and aforementioned clinical outcomes; (f) composited outcomes rather than separate outcomes were provided.
2.2. Data abstraction
The following data were abstracted by three reviewers (LY, HYM and HY) from all included eligible studies: author, publication year, country, study type, sample size and valid participants numbers, study period, mean age or median age, age range, numbers of males, measure of obesity, numbers of participants with obesity, and defined clinical outcomes. Risks of severe COVID-19 were presented as OR and 95% CI for cohort studies with BMI-defined obesity and standardized mean difference (SMD) and 95%CI for controlled studies with VAT-defined excessive adiposity.
2.3. Quality assessment
The Newcastle–Ottawa Scale (NOS) was used in this study to assess quality of all included cohort studies. Since age is a well-established risk factor for COVID-19 severity, we selected age as the most important factor for comparability of cohorts on the basis of the design or analysis. Studies with more than 7 stars, 5–7 stars, and 0–4 stars had high, moderate, and low quality, respectively.
2.4. Statistical analysis
For included studies that provided data about BMI-defined obesity group and corresponding clinical outcomes rather than OR and 95%CI, we performed Chi-square test and risk estimation statistics using SPSS 22.0 to calculate P value, related OR and 95%CI that applied to univariate analysis. For each study that provided age-specific or BMI-specific OR and 95%CI for association between obesity and defined outcomes, we conducted the effect size combination for all age subgroups as well as all obesity groups and non-obesity group using Stata/SE 12.0. We then conducted the effect size combination for all included studies. Pooled OR and 95%CI estimates were calculated using Stata/SE 12.0 to address the relationship between BMI-defined obesity and the risk of defined outcomes among patients with COVID-19. For continuous outcome variables, SMD is a measure of distance between two group means in terms of one or more variables and used as a summary statistic when outcome from each study is measured using several different scales [16]. Since all defined-indicators of quantification of VAT among studies were accepted in our meta-analysis, SMD was used as a summary statistic for included studies to evaluate VAT difference between severe COVID-19 and non-severe COVID-19 groups. The I 2 statistic was used to describe the degree of heterogeneity among the studies. I 2 > 50% was considered as high heterogeneity and this allowed us to use the random-effects model, while I 2<50% allowed us to run the fixed-effects model. Potential publication bias was evaluated by visual inspection of funnel plots. Egger's tests were used to assess the symmetry of a funnel plot. The level of statistical significance was defined as P < 0.05.
3. Results
3.1. Included studies
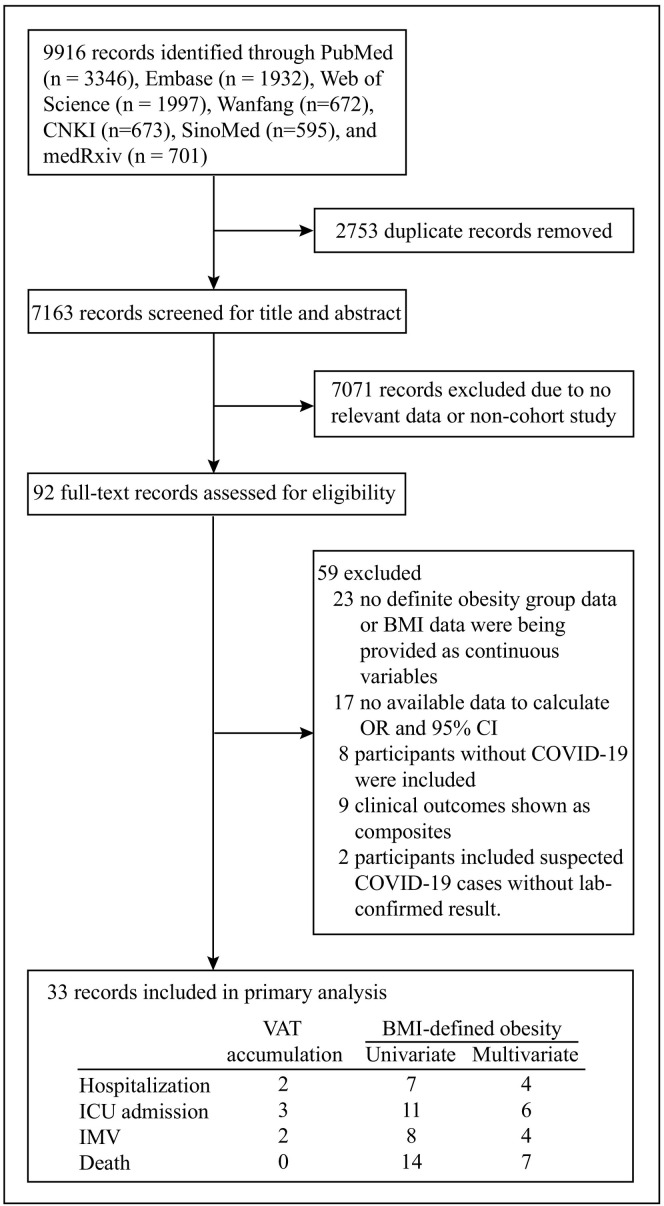
A total of 9, 916 articles were identified using the search strategies (Fig. 1 ). After removing 2, 753 duplicated records, 7, 163 studies were then screened for title and abstract and 7, 071 were excluded due to having no relevant data or being a non-cohort study. The remaining 92 articles were fully reviewed and 33 studies which met inclusion criteria were included. Among 33 included studies, 30 studies were analyzed to assess the association between BMI and severe COVID-19 while the remaining 3 studies were analyzed to evaluate the association between VAT and severe COVID-19. From 92 full-text articles that were assessed for eligibility, we excluded 59 studies (Supplementary appendix A) owing to no available definite obesity group data or BMI data being provided as continuous variables (n = 23), no available data to calculate OR and 95% CI (n = 17), where the study included participants without COVID-19 (n = 8), where participants of a study included suspected cases of COVID-19 which were not confirmed by SARS-CoV-2 diagnostic tests (n = 2) or where clinical outcomes of the study shown as composited and could not be analysis separately (n = 9). Thirty included studies which reported BMI as a variable, were assigned to different groups according to various specified outcomes. For univariate analysis, there were seven studies included to estimate the risk of hospitalization, eleven studies to estimate the risk of ICU admission, eight studies to estimate the need for IMV and fourteen to estimate the risk of death. For multivariate analysis, there were four studies included to estimate the risk of hospitalization, six studies to estimate the risk of ICU admission, four studies the risk of requiring IMV and seven studies the risk of death. We identified one study from the Bronx borough of New York City reporting a positive association between BMI ≥ 35 kg/m2 and worse in-hospital outcomes among 200 patients with COVID-19 [12]. In this retrospective cohort study, BMI ≥ 35 kg/m2 (reference: BMI 25–34 kg/m2) was independently associated with increased risks of intubation (OR: 3.87; 95%CI: 1.47, 10.18; P = 0.006) and in-hospital mortality (OR: 3.78; 95%CI: 1.45, 9.83; P = 0.006). However, this valuable study provided no available data on defined obesity group (BMI ≥ 30 kg/m2) and therefore was not included in our meta-analysis. Among the other three included studies reporting VAT as variable, there were two studies included to estimate the risk of hospitalization, three studies the risk of ICU admission, two studies the risk of needing IMV and no studies to estimate the risk of death. We identified only one article reporting an association between VAT area and death related to COVID-19 [15]. However, this research was finally excluded from our study as there was no available data of VAT area for the survivor group.
Fig. 1.
Flowchart of screened and included studies.
In the box of included records, column headings represent obesity condition including VAT accumulation and BMI-defined obesity; Rowheadings represent clinical outcomes including hospitalization, ICU admission, IMV and death. Abbreviation: CNKI, Chinese National Knowledge Infrastructure; BMI, body mass index; OR, odds ratio; 95%CI, 95% confidence interval; COVID-19, Coronavirus Disease 2019; VAT, visceral adipose tissue; ICU, intensive care unit; IMV, invasive mechanical ventilation.
3.2. Characteristics and quality assessment
The included studies involved 9 countries over the world including USA, Italy, China, Spain, The state of Kuwait, Mexico, France, Switzerland and Greece. A total of 45, 650 participants were finally included into analysis. Nearly two third of the studies (18/33) were from USA, the current epicenter of the coronavirus pandemic. A retrospective study design was used in the majority of included studies (87.9%, 29/33). We identified five studies, which didn't report their study type as retrospective studies according to their study methods [7,9,[17], [18], [19]]. Only one of the included studies was designed as a retrospective and prospective study [20]. Patients mainly participated in these studies between February to May. The participants in 20 studies were all adults, over 18 years of age, while 11 studies did not report the age range of their participants in detail. Only one study included exclusively children with a median age of 13.1 (0.4–19.3) [21]. Except for this study, the median age of participants ranges from 40.5 (31.5–52.1) to 72 (60–80) years, with fourteen studies reporting a mean age or no statistical description for their age range. Obesity criteria among 24 studies were defined as a level of BMI of 30 kg/m2 or more. One study from China defined a BMI of 28 kg/m2 or more as obesity in accordance to obesity criteria of Chinese adults [9], another study from Italy defined a BMI of over 29 kg/m2 as obesity [22]. It should be noted that one study emphasized that the World Health Organization defined obesity as abnormal or excessive fat accumulation that presents a risk to health [14]. No obesity definition was given in six studies [17,20,[23], [24], [25], [26]]. The percentage of people with obesity among valid participants of all but one of the included studies ranged from 10.9% to 61.3%, with only one study below 10% (Table 1 ). Among three studies that aimed to evaluate the association between VAT and severe COVID-19, a CT measurement was performed to quantify VAT. One study defined VAT as the greatest distance between the inner muscular wall and the anterior liver surface and therefore assessed VAT amount by thickness [25], whereas two other studies [14,26] assessed VAT amount by area (Table B. Supplementary appendix B). In the two studies, VAT content was evaluated at different abdominal levels of CT scan: the first slice where lung bases were no longer visible at the thoracoabdominal level in one study [14] and the axial slice at the superior end plate of L3 vertebral body in the other study [26].
Table 1.
Characteristics of included studies.
| Study author, year | Country | Study type | Sample size | No. of valid participants | Median age | Age range | Male (%) | Study period | Measure of Obesity | No. of participants with obesity (%) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Giacomelli et al., 2020 [11] | Italy | Prospective | 233 | 233 | 61(50–72) | 18–95 | 161(69.1) | 21/2–19/5 | BMI ≥ 30 | 38(16.3) | Death |
| Borobia et al., 2020 [17] | Spain | Retrospective | 2226 | 2226 | 61 (46–78) | 18–90 | 1074 (48.2) | 25/2–19/4 | NA | 242(10.9) | Death |
| Kalligeros et al., 2020 [68] | USA | retrospective | 103 | 103 | 60 (50–72) | ≥18 | 63 (61.2) | 17/2–5/4 | BMI ≥ 30 | 49(47.5) | ICU admission, IMV |
| Chao et al., 2020 [21] | USA | Retrospective | 46 | 46 | 13.1(0.4–19.3) | 1 month to 21 years | 31(67.4) | 15/3–13/4 | BMI>30 | 12(26.1) | ICU admission |
| Giorgi Rossi et al., 2020 [23] | Italy | Prospective | 2653 | 2407 | 63.2 | NA | 1328(50.1) | 27/2–2/4 | NA | 65(2.7) | Hospitalization, death |
| Goyal et al., 2020 [69] | USA | Retrospective | 393 | 380 | 62.2(48.6–73.7) | ≥18 | 238 (60.6) | 5/3–27/3 | BMI ≥ 30 | 136(35.8) | IMV |
| Argenziano et al., 2020 [70] | USA | Retrospective | 1000 | 781 | 63.0(50–75) | ≥18 | 596(59.6) | 1/3–5/4 | BMI > 30 | 352(41.6) | ICU admission |
| Al-Sabah et al., 2020 [71] | The State of Kuwait | Retrospective | 1158 | 727 | 40.5(31.5–52.1) | NA | 945 (81.6) | 24/2–7/4 | BMI ≥ 30 | 148(20.4) | ICU admission |
| Petrilli et al., 2020 [72] | USA | Prospective | 5279 | 5040 | 54 (38–66) | ≥19 | 2615 (49.5) | 1/3–8/4 | BMI ≥ 30 | 1865(37.0) | Hospitalization |
| Mejia-Vilet et al., 2020 [73] | Mexico | Prospective | 329 | 329 | 49 (41–60) | >18 | 211 (64) | 16/3–8/5 | BMI > 30 | 132(40.1) | ICU admission |
| Hur et al., 2020 [74] | USA | Retrospective | 486 | 486 | 59(47–69) | ≥18 | 271(55) | 1/3–8/4 | BMI ≥ 30 | 259(53.3) | IMV |
| Robilotti et al., 2020 [75] | USA | Retrospective | 423 | 423 | NA | NA | 212 (50) | 10/3–7/4 | BMI ≥ 30 | 130(30.7) | Hospitalization |
| Carrillo-Vega et al. [24] | Mexico | Retrospective | 10,544 | 9946 | 48.15 ± 14.35§ | NA | 6082 (57.7) | 27/2–23/4 | NA | 2053(20.64) | Hospitalization, death |
| Simonnet et al., 2020 [8] | France | Retrospective | 124 | 124 | 60(51–70) | NA | 90 (72.6) | 27/2–5/4 | BMI > 30 | 59(47.6) | IMV |
| Shekhar et al., 2020 [20] | USA | Retrospective& prospective | 50 | 50 | 55.5(20–85) | ≥18 | 23(46) | 19/1–24/4 | NA | 20/39(51) | ICU admission |
| Klang et al., et al., 2020 [40] | USA | Retrospective | 3406 | 3406 | NA | ≥18 | 1961(57.6) | 1/3–17/5 | BMI ≥ 30 | 1231(36.1) | Death |
| Cai et al., 2020 [9] | China | Retrospective | 383 | 383 | NA | ≥18 | 183(47.5) | 11/1–26/3 | BMI ≥ 28 | 41(10.7) | ICU admission, death |
| Regina et al., 2020 [76] | Switzerland | Retrospective | 200 | 200 | 70(55–81) | ≥18 | 120 (60) | 1/3–25/3 | BMI > 30 | 54(27) | IMV |
| Lighter et al., 2020 [10] | USA | Retrospective | 3615 | 3615 | NA | ≥18 | NA | 3/3–4/4 | BMI ≥ 30 | 547(15.1) | ICU admission |
| Petrilli et al., 2020 [7] | USA | Retrospective | 4103 | 4103 | 52(36–65) | NA | 2072 (50.5) | 1/3–2/4 | BMI ≥ 30 | 1100(26.8) | Hospitalization |
| Kim et al., 2020 [18] | USA | Retrospective | 2491 | 2491 | 62(50–75) | ≥18 | 1326(53.2) | 1/3–2/5 | BMI ≥ 30 | 1154/2332(49.7) | ICU admission, death |
| Gaibazzi et al., 2020 [22] | Italy | Retrospective | 279 | 279 | 72 (60–80) | NA | 169(61) | 5/3–15/3 | BMI>29 | 29/181(16) | Death |
| Ebinger et al., 2020 [19] | USA | Retrospective | 442 | 442 | 52.7 ± 19.7§ | NA | 256(58) | 26/2–21/3 | BMI ≥ 31 | 71(16.1) | Hospitalization, ICU admission, IMV |
| Daniel et al., 2020 [77] | USA | Retrospective | 172 | 172 | 53(33.5–68) | NA | 96(55.8) | 12/3–8/5 | BMI>30 | 89(51.7) | Death |
| Murillo-Zamora et al., 2020 [78] | Mexico | Retrospective | 5393 | 5393 | NA | ≥18 | 3432(63.6) | 4/3–5/5 | BMI ≥ 30 | 1197(22.2) | Death |
| Halvatsiotis et al., 2020 [79] | Greece | Retrospective | 90 | 86 | 65.5 (56–73) | NA | 72(80) | 10/3–13/4 | BMI>30 | 30(34.4) | Death |
| Rottoli et al., 2020 [80] | Italy | Retrospective | 516 | 482 | 66.2 ± 16.8§ | ≥18 | 302(62.7†) | 1/3–20/4 | BMI ≥ 30 | 104(21.6) | ICU admission, death |
| Steinberg et al., 2020 [81] | USA | Retrospective | 210 | 210 | NA | 18–45 | NA | 8/3–4/4 | BMI>30 | NA | Hospitalization, IMV, death |
| Pettit et al., 2020 [35] | USA | Retrospective | 238 | 238 | 58.5 ± 17§ | NA | 113(47.5) | 1/3–18/4 | BMI ≥ 30 | 146(61.3) | ICU admission, IMV, death |
| Nakeshbandi et al., 2020 [82] | USA | Retrospective | 504 | 504 | 68 ± 15§ | ≥18 | 263(52) | 10/3–13/4 | BMI ≥ 30 | 215(30) | IMV, death |
| Chandarana et al., 2020 [26] | USA | Retrospective | 51 | 51 | 59.8 ± 14.9§ | 20–88 | 38(70.4) | 19/3–19/4 | VAT deposition | NA | Hospitalization, ICU admission, IMV |
| Battisti et al., 2020 [25] | Italy | Retrospective | 441 | 144 | 60.3 ± 17.0§ | NA | NA | 26/2–6/4 | VAT deposition | NA | ICU |
| Watanabe et al., 2020 [14] | Italy | Retrospective | 150 | 150 | 64.15 ± 15.69§ | 22–97 | 97(64.7) | 1/3–31/3 | VAT deposition |
NA | Hospitalization, ICU admission, IMV |
BMI = Weight (kg)/Height2(m2) and is expressed in units of kg/m2. Abbreviation: BMI, body mass index; NA, not available; ICU, intensive care unit; IMV, invasive mechanical ventilation; VAT, visceral adipose tissue.
§ Statistical description of age was presented as Mean ± Standard Deviation.
¶ We identified these five studies, which didn't report their study type as retrospective studies according to their study methods.
† Proportion of male among total valid participants.
Using the NOS method, studies included in the present analysis had an average score of 8, with 22 studies achieved 8 or 9 scores and were then considered as cohort study of high quality. Eleven studies achieved scores of 5 to 7 and thus were considered as moderate quality (Table 2 ).
Table 2.
Quality assessment of included studies using the Newcastle–Ottawa Scale (NOS).
| Selection |
Comparability |
Outcome |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Study ID | Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertain-ment of exposure | Demonstration that outcome of interest was not present- at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |
| Giacomelli et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Borobia et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Kalligeros et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Chao et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |||
| Giorgi Rossi et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Goyal et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |||
| Argenziano et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |||
| Al-Sabah et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Petrilli et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Mejia-Vilet et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Hur et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Robilotti et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||
| Carrillo-Vega et al | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |||
| Simonnet et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Shekhar et al | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |||
| Klang et al., et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Cai et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Regina et al | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Lighter et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ||||
| Petrilli et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Kim et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Gaibazzi et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Ebinger et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Daniel et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Murillo-Zamora et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Halvatsiotis et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||
| Rottoli et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Steinberg et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ||||
| Pettit et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Nakeshbandi et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Chandarana et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||
| Watanabe et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Battisti et al., 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||
3.3. Univariate analysis of association between BMI-defined obesity and severe COVID-19
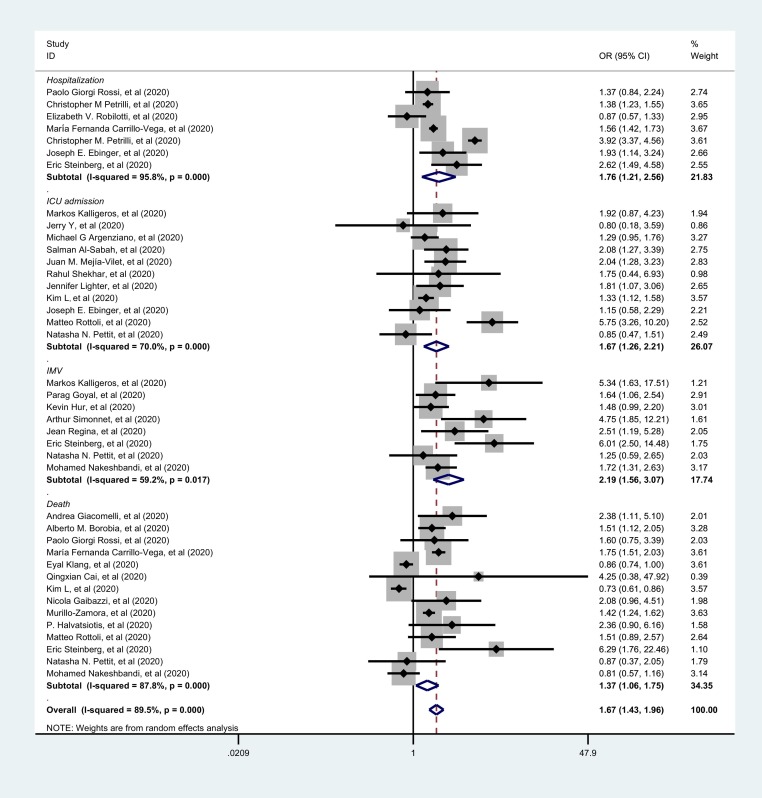
Pooled effects of univariate analysis of included studies indicated that BMI-defined obesity increased risk of severe disease among patients with COVID-19, as shown in Fig. 2 . COVID-19 patients with obesity had a significantly increased risk of needing hospitalization (OR: 1.76, 95% CI: 1.21, 2.56, P = 0.003) and ICU admission (OR: 1.67, 95% CI: 1.26, 2.21, P<0.001). Similarly, obesity also increased risk of death for patient with COVID-19 (OR: 1.37, 95% CI: 1.06, 1.75, P = 0.014). Moreover, obesity was in particular found to be associated with an over twofold significantly increased risk of IMV among patients with COVID-19 (OR:2.19, 95%CI: 1.56, 3.07, P<0.001). The overall risk estimation of univariate analyses showed significant OR of obesity with COVID-19 severity: 1.67 (95%CI: 1.43, 1.96, P<0.001). Furthermore, since Paleodimos and colleagues reported the importance of severe obesity as a critical component to COVID-19 severity in the cohort of the Bronx borough of New York City [12], we included their study in our meta-analysis and performed a summary estimation (Table C. Supplementary appendix C). The pooled OR and 95%CI estimates did not change appreciably: an OR of 2.22 (95%CI: 1.62, 3.03; P<0.001) by univariate association between BMI-defined obesity and IMV requirement among COVID-19 patients, an OR of 1.41 (95%CI: 1.11, 1.81; P = 0.006) between BMI-defined obesity and COVID-19 mortality and an overall OR of 1.70 (95%CI: 1.45, 1.99; P<0.001) between BMI-defined obesity and the risk of COVID-19 severity.
Fig. 2.
Forest plots of univariate association between BMI-defined obesity and the risk of COVID-19 severity using the random-effects model.
Clinical outcome of each subgroup is marked in italics. The gray squares show the estimated effect of each single study and their sizes reflected the weight of each single study on the summary effect. The larger the size, the greater the weight. The diamonds represent the overall summary effects with their widths reflecting the length of the 95% CI. A wider diamond means a wider 95% CI. The horizontal black lines through the gray squares also represent the length of the 95% confidence interval of individual studies. The longer the line, the wider the 95% CI. The solid vertical black line is the line of no effect. The region to the left of the line of no effect indicates no association while the region to the right indicates association. When the diamond touches the solid vertical black line, this indicates no statistical difference. The dotted black line is the line of the overall summary effect. Subtotal effect estimate results and the overall results are marked in bold. I-squared indicates the degree of heterogeneity within the studies. Abbreviation: OR, odds ratio; 95%CI, 95% confidence interval; ICU, intensive care unit; IMV, invasive mechanical ventilation; COVID-19, Coronavirus Disease 2019.
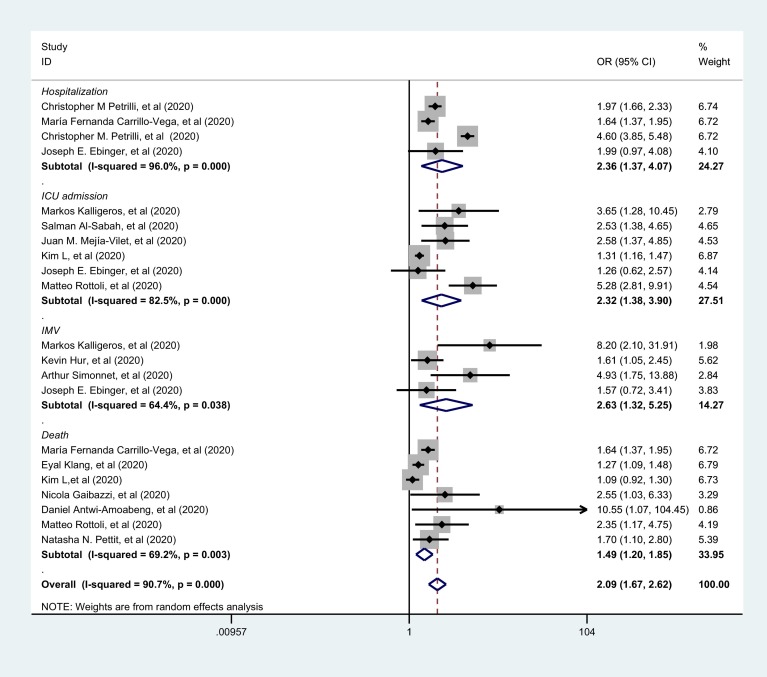
3.4. Multivariate analysis association between BMI-defined obesity and COVID-19 severity
Pooled summary effects of multivariate analysis of included studies also suggested that BMI-defined obesity increased risk of severe disease and death among patients with COVID-19, as shown in Fig. 3 . After adjusting for covariate factors, COVID-19 patients with obesity were also at a high risk of needing hospitalization (OR: 2.36, 95%CI: 1.37, 4.07, P = 0.002) and ICU admission (OR: 2.32, 95%CI: 1.38, 3.90, P = 0.001). Compared to univariate analysis, and after multivariate adjustment, obesity added a nearly 3-fold increased risk of needing IMV among patients with COVID-19 (OR: 2.63, 95%CI: 1.32, 5.25, P = 0.006). However, in multivariate analysis, there were only minor changes of increased risk of death that obesity added to patients with COVID-19(OR: 1.49, 95%CI: 1.20, 1.85, P<0.001). The overall risk estimation of multivariate analyses showed a significant OR of severe COVID-19 with obesity: 2.09 (95%CI: 1.67, 2.62, P<0.001). When the Paleodimos study were included in our meta-analysis, the pooled OR and 95%CI estimates slightly increased (Table C. Supplementary appendix C): an OR of 2.79 (95%CI: 1.54, 5.04; P = 0.001) by multivariate association between BMI-defined obesity and IMV requirement among COVID-19 patients, an OR of 1.57 (95%CI: 1.25, 1.98; P<0.001) between BMI-defined obesity and COVID-19 mortality and an overall OR of 2.17 (95%CI: 1.74, 2.70; P<0.001) between BMI-defined obesity and the risk of COVID-19 severity.
Fig. 3.
Forest plots of multivariate association between BMI-defined obesity and the risk of COVID-19 severity using the random-effects model.
Clinical outcome of each subgroup is marked in italics. The gray squares show the estimated effect of each single study and their sizes reflect the weight of each single study on the summary effect. The larger the size, the greater the weight. The diamonds represent the overall summary effects with their widths reflecting the length of the 95% CI. A wider diamond means a wider 95% CI. The horizontal black lines through the gray squares also represent the length of the 95% CI of individual studies. The longer the line, the wider the 95% CI. The solid vertical black line is the line of no effect. The region to the left of the line of no effect indicates no association while the region to the right indicates association. When the diamond touches the solid vertical black line, this indicates no statistical difference. The dotted black line is the line of the overall summary effect. Subtotal effect estimate results and the overall results are marked in bold. I-squared indicates the degree of heterogeneity within the studies. Abbreviation: OR, odds ratio; 95%CI, 95% confidence interval; ICU, intensive care unit; IMV, invasive mechanical ventilation; COVID-19, Coronavirus Disease 2019.
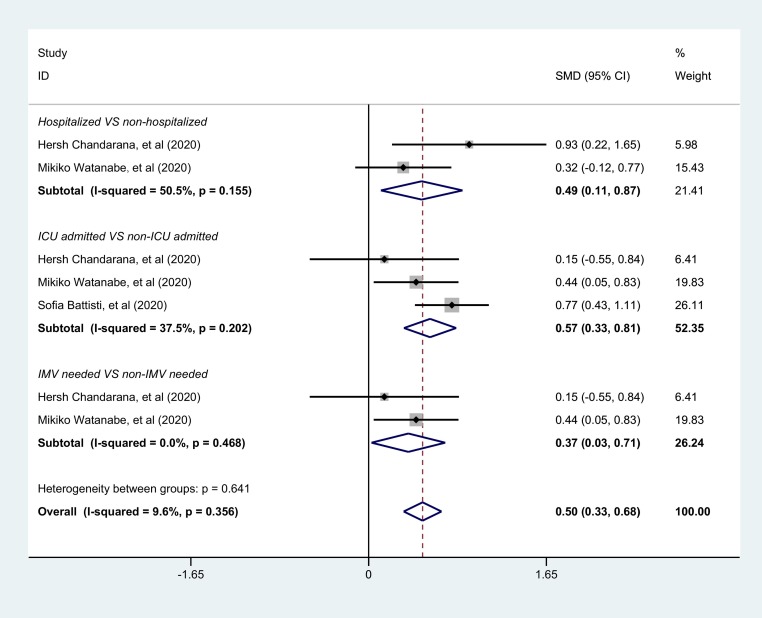
3.5. Increased VAT accumulation among patients with severe COVID-19
Compared to the non-severe COVID-19 group, VAT accumulation levels were significantly higher in COVID-19 patients with a severe condition. For the hospitalized group versus those not hospitalized, the SMD was 0.49 (95% CI: 0.11, 0.87; P = 0.011), For the ICU admission group versus those not admitted to ICU, the SMD was 0.57 (95% CI: 0.33, 0.81; P<0.001) and for the IMV group versus those not needing IMV, the SMD was 0.37 (95% CI: 0.03, 0.71; P = 0.035) (Fig. 4 ). The overall estimated effect was statistically significant (SMD = 0.50, 95% CI: 0.33, 0.68; P<0.001) (Fig. 4), indicating that the more VAT accumulation a COVID-19 patient had, the more severe the clinical condition they might develop.
Fig. 4.
Forest plots of VAT amount between severe group and non-severe group among COVID-19 patients using the fixed-effects model.
The gray squares show the estimated effect of each single study and their sizes reflect the weight of each single study on the summary effect. The larger the size, the greater the weight. The diamonds represent the overall summary effects with their widths reflecting the length of the 95% CI. A wider diamond means a wider 95% CI. The horizontal black lines through the gray squares also represent the length of the 95% CI of individual studies. The longer the line, the wider the 95% CI. The solid vertical black line is the line of no effect. The region to the left of the line of no effect indicates a lower mean value for the experimental group versus the control group while the region to the right indicates a higher mean value for the experimental group versus the control group. When the diamond touches the solid vertical black line, this indicates no statistical difference. The dotted black line is the line of the overall summary effect. Subtotal effect estimate results and the overall results are marked in bold. I-squared indicates the degree of heterogeneity within the studies. Abbreviation: SMD, Standardized Mean Difference; 95%CI, 95% confidence interval; ICU, intensive care unit; IMV, invasive mechanical ventilation; VAT, visceral adipose tissue;COVID-19, Coronavirus Disease 2019.
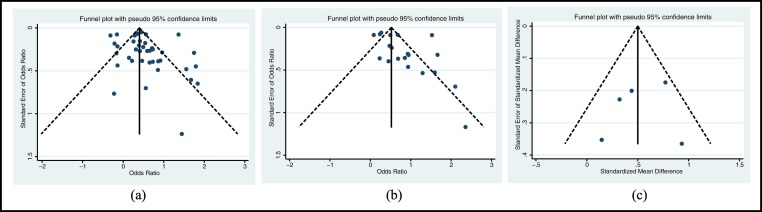
3.6. Assessment of publication bias
No significant publication bias was shown in the funnel plots (Fig. 5 .) and the result was confirmed by Egger's test (Egger's tests: Fig. 5a. P = 0.332; Fig. 5b. P = 0.132; Fig. 5c. P = 0.438).
Fig. 5.
Funnel plot of included studies for publication bias.
a. Funnel plot of included studies using univariate analysis between BMI-defined obesity and COVID-19 severity; b. Funnel plot of included studies using multivariate analysis between BMI-defined obesity and COVID-19 severity; c. Funnel plot of included studies that assessed the association between VAT accumulation and severe COVID-19.
4. Discussion
Our study confirms that obesity may increase the risks of hospitalization, ICU admission, the necessity for IMV, and death among patients with COVID-19. An early systematic review addressed that obesity was a predictor for a worse prognosis of COVID-19 with a total of three cohort studies included [27]. Previous meta-analysis studies indicated that COVID-19 patients with higher BMI were at a greater risk of medical complications [28], ICU admission [29,30], IMV intervention [29,30], death [31,32] and poor composite outcomes [31,33,34]. Findings in our study are consistent with those reports, suggesting a positive relationship between higher BMI and increased risk of disease severity among patients with COVID-19.
In terms of method, both univariate and multivariate analyses of the association between high BMI and clinical outcomes were performed in our meta-analysis to provide a contrast among study results, which differs from previous similar meta-analyses. Moreover, previous meta-analyses focused mainly on the association between BMI-defined obesity and COVID-19 severity. However BMI does not reflect the distribution of body fat and therefore these research analyses fail to demonstrate the impact of excess body fat in different parts of the body on COVID-19 severity. To the best of our knowledge, this is the first meta-analysis to identify a positive relationship between high VAT accumulation and severe COVID-19.
Obesity prevalence among hospitalized COVID-19 patients can reach up to 61.3%, according to an American study from our included articles [35]. In addition to the impact of obesity on the risk of hospitalization, this may also due to high prevalence of obesity in American population. The prevalence of obesity among adults in the USA was 42.4%, with severe obesity in 9.2% in 2017–2018 [36]. By 2016, obesity had reached 27.8% in the UK, 19.9% in Italy, 6.2% in China, 4.7% in Korea, 3.9% in India, 22.1% in Brazil, 23.8% in Spain, 21.6% in France and 28.9% in Mexico [37]. Of particular note is that BMI threshold differs between Asian and Caucasian populations, and health risks among these two groups can be different at any given BMI. Despite the lower prevalence of BMI-defined obesity, awareness of a potentially similar obesity-related risk of COVID-19 should be not be delayed by Asian countries.
Since older age has been well recognized as an important risk factor of suffering a severe condition of COVID-19, we should be cautious about applying the findings of our meta-analysis to children. Most infected children appear to experience more favorable outcomes than adults [38]. The study we included that examined children had a relatively small subject number and did not find an association between obesity and increased likelihood of pediatric intensive care unit admission [21]. However, a significant inverse correlation between age and BMI was reported among 265 patients admitted to ICU, meaning that obesity could shift severe COVID-19 disease to younger ages [39]. On the other hand, two studies we included found that the effect of BMI ≥30 kg/m2 on COVID-19 severity or death among adults was age-dependent [10,40]. Compared to younger patients, older COVID-19 patients with BMI ≥ 30 kg/m2 appeared to develop a less severe condition [10,40]. Nevertheless, it's worth noting that the gradient of risk of severe COVID-19 in relation to BMI might be more gradual among older patients when compared to younger individuals [41]. This may be attributed to the fact that BMI is a less accurate predictor of excess fat in older adults with lower muscle mass, together with a shift from subcutaneous fat to VAT and increased relative fat mass among them [14]. A more precise measurement of excess fat may help predict more reliable health risks in this group with obesity. The positive relationship between VAT and severe COVID-19 in our meta-analysis may provide an important insight.
The underlying mechanism by which obesity increases the risk of severe covid-19 remains unknown. Previous research has shown that obesity was related to a worse outcome as a result of infection and disease progression for certain kinds of infectious virus diseases, such as influenza in the 1918 “Spanish” influenza pandemic [42,43], the 1957 pandemic, the 1968 pandemic and the 2009 Influenza A virus (IAV) H1N1 pandemic [44,45]. People with obesity tend to have respiratory dysfunction at various levels [46] and may be mildly hypoxaemic [47]. A greater oxygen cost of breathing was needed for patients with obesity when compared to those without obesity, even at rest [47]. In a recently published meta-analysis, dyspnea rather than fever was shown to be significantly associated with the risk of mortality among COVID-19 patients [48]. One study we included found that BMI ≥ 30 kg/m2 were associated with the risk of hypoxemia upon hospital admission among patients with COVID-19 (OR: 1.7, 95%CI: 1.3, 2.1; P < 0.0005) [35]. A BMI ≥ 35 kg/m2 was even a significant predictor for increasing oxygenation requirement in a cohort of COVID-19 patients in the Bronx borough of New York City [12]. In the children-included study [21], shortness of breath was found to be the only clinical symptom that was associated with pediatric intensive care unit admission. Patients diagnosed with obesity hypoventilation syndrome developed typical hypoxaemia and hypercapnia, and may suffer a higher risk of severe condition when infected with SARS-CoV-2 [49]. Therefore, obesity-related hypoxaemia might be an important contributor to COVID-19 severity among those with obesity. This may explain why COVID-19 patients with obesity were at a greater risk of needing IMV, as shown by results in our study.
Obesity also increases the risk of many common non-communicable diseases such as diabetes mellitus, cardiovascular disorders, cancers and non-alcoholic fatty liver disease, and often co-exists with them in a single individual. These co-existing co-morbidities are considered to increase the likelihood of severe illness from COVID-19 for people with obesity [[50], [51], [52]]. Excessive adipose tissue including ectopic fat may serve as reservoirs for angiotensin-converting enzyme 2 (ACE2) and microbes such as coronavirus, influenza A virus and Mycobacterium tuberculosis [53]. Beyond disease severity, obesity increased the duration of influenza A virus shedding to hasten virus spreading mainly for person-to-person transmission [54]. Moreover, college volunteers with symptomatic seasonal influenza who had higher BMI have been found to generate more infectious aerosols [55]. These findings may also extend to COVID-19.
Above all, combined adipose tissue-mediated immune and metabolic dysfunctions might play a key role in the pathophysiological pathways that lead obesity to influence COVID-19 prognosis [46,56,57]. Low-grade systemic inflammation and increasing insulin resistance commonly exists in people with obesity [58,59]. and this immune and metabolic phenomena is strongly associated with presence of excess VAT [60]. Excess VAT is believed to be the main culprit in the inflammatory diseases of obesity [60], which in turn might induce severe complications on top of the viral infection itself, such as development of thrombosis [56]. Visceral obesity-related impaired immune response can also lead to systemic metabolic dysfunction [43,56] and increase risks of metabolic disorders and cardiovascular diseases, as well as their complications [[61], [62], [63]]. Furthermore, while BMI on its own does not reflects any particular distribution of body fat, VAT is a marker of increased ectopic fat that might contribute to increased atherosclerosis and cardiometabolic risk [64]. Excessive visceral adiposity may provide additional important information about COVID-19 risk, which is not captured in BMI. Evidence of value from two recent studies, which were not included in our analysis due to our study design and eligibility criteria, suggests that visceral adiposity increases the likelihood of severe COVID-19 [15,65]. Central obesity is defined as a state of excessive VAT accumulation [66]. Patients with central obesity evidenced by waist circumference or waist-to-hip ratio were also found to be more likely to develop severe COVID-19 (P<0.001) in a large population-based cohort [67]. Our primary analysis also demonstrates a more VAT accumulation among patients with severe COVID-19. For the sake of comparison, we also extracted data on subcutaneous adipose tissue (SAT) from the included 3 controlled studies with VAT-defined adiposity, and performed a separate meta-analysis to evaluate SAT accumulation with the risk of severe COVID-19 (Table D & Fig. D. Supplementary appendix D). The overall estimated SMD was −0.05 (95% CI: −0.22, 0.13; P = 0.601), indicating that there might have no significant difference of SAT accumulation between severe group and non-severe group among COVID-19 patients. Therefore, compared to SAT, VAT accumulation might be a stronger contributor to COVID-19 severity.
The present study assesses the risk of severe COVID-19 with obesity, using not only BMI but also VAT measures, and this is the main strength of our study. There are also several limitations to be acknowledged. First, some participants we included might overlap, because some of our included studies are from the same affected area or city and both single-center and multi-center studies were included in our meta-analysis. Second, despite our study seeking to investigate the role of VAT accumulation in the risk of severe COVID-19, diagnostic cut-off points by VAT amount for obesity of the three included studies could not be set in our meta-analysis since no accepted standards of diagnostic criteria by VAT quantification for obesity were available. Third, while fat mass can be classified as VAT, SAT, ectopic fat (intra-muscular fat, myocardial steatosis, fatty liver, atheroma, et al), and blood lipids (dyslipidemia) we have only evaluated the impact of VAT and SAT on COVID-19 prognosis. A comprehensive assessment of the relationship between excess fat mass and the risk of severe COVID-19 will require a thorough literature search and extensive future investigation. Fourth, in our study, the effects of combining all included studies could lead to a bias due to the adjusted confounders for BMI or obesity state varying between the included studies, particularly in the absence of adjusting for some unrecognized factors. Last, since the vast majority of included studies were designed as retrospective, a causal relationship between obesity and COVID-19 severity and death should not be identified.
5. Conclusions
Our study suggests that obesity increases risk for hospitalization, ICU admission, need for IMV and death among patients with COVID-19. Further, excessive visceral adiposity appears to be associated with severe COVID-19 outcomes. The clinical outcomes of communicable disease such as COVID-19 might also depend on obesity status. These findings emphasize the need for effective actions by individuals, the public and governments to increase awareness of the risks resulting from obesity and how these are heightened in the current global pandemic.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81471054, 81660150), the Innovation Project of Guangxi Graduate Education (JGY2015128) and Seeding Fund of the Center for Diabetic Systems Medicine, Guangxi Key Laboratory of Excellence (203030401902). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Yi Huang: Data curation, Formal analysis, Writing - original draft. Yao Lu: Data curation, Validation, Investigation. Yan-Mei Huang: Data curation. Min Wang: Formal analysis. Wei Ling: Formal analysis. Yi Sui: Writing - review & editing. Hai-Lu Zhao: Conceptualization, Methodology, Writing - review & editing.
Declaration of competing interest
We declare no competing interests.
Acknowledgments
Acknowledgement
We are grateful to Dr. Phil Griffiths for his work revising the written English in our text.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metabol.2020.154378.
Appendix A. Supplementary data
Appendix A-List of 59 disqualified papers
Appendix B-Table and reference list of three studies reporting VAT accumulation
Appendix C-Different effects between inclusion and exclusion of the study by Palaiodimos and co-authors
Appendix D-Table and forest plots of the three studies reporting SAT accumulation
References
- 1.World Health Organization Coronavirus. 2020. https://www.who.int/health-topics/coronavirus#tab=tab_1
- 2.World Health Organization Coronavirus disease 2019. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 3.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19[published online ahead of print, 2020 May 15] N Engl J Med. 2020 doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 4.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Obesity and overweight. 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 6.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of general practitioners research and surveillance Centre primary care network: a cross-sectional study[published online ahead of print, 2020 May 15] Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L.F., Chernyak Y. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. Preprint at medRxiv. 2020 doi: 10.1101/2020.04.08.20057794. [DOI] [Google Scholar]
- 8.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q. Obesity and COVID-19 severity in a designated Hospital in Shenzhen. China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 10.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx. New York Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M. Visceral fat shows the strongest association with the need of intensive Care in Patients with COVID-19[published online ahead of print, 2020 Jul 23] Metabolism. 2020:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y., Ding L., Zou X., Shen Y., Hu D., Hu X. Visceral Adiposity and High Intramuscular Fat Deposition Independently Predict Critical Illness in Patients with Sars-COV-2 [published online ahead of print, 2020 Jul 17] Obesity (Silver Spring) 2020 doi: 10.1002/oby.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murad M.H., Wang Z., Chu H., Lin L. When continuous outcomes are measured using different scales: guide for meta-analysis and interpretation. BMJ. 2019;364:k4817. doi: 10.1136/bmj.k4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borobia A.M., Carcas A.J., Arnalich F., Alvarez-Sala R., Montserrat J., Quintana M. A cohort of patients with COVID-19 in a major teaching hospital in Europe. Preprint at medRxiv. 2020 doi: 10.1101/2020.04.29.20080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim L, Garg S, O'Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Interim Analysis of Risk Factors for Severe Outcomes among a Cohort of Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Preprint at medRxiv 2020. 10.1101/2020.05.18.20103390. [DOI]
- 19.Ebinger J.E., Achamallah N., Ji H., Claggett B.L., Sun N., Botting P. Pre-existing traits associated with Covid-19 illness severity. Preprint at medRxiv. 2020 doi: 10.1101/2020.04.29.20084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shekhar R., Upadhyay S., Sheikh A., Atencio J., Kapuria D. Early experience with COVD-19 patients at tertiary care teaching hospital in southwestern United States. Preprint at medRxiv. 2020 doi: 10.1101/2020.05.15.20094284. [DOI] [PubMed] [Google Scholar]
- 21.Chao J.Y., Derespina K.R., Herold B.C., Goldman D.L., Aldrich M., Weingarten J. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 (COVID-19) at a tertiary care medical center in New York City. J Pediatr. 2020;223:14–19. doi: 10.1016/j.jpeds.2020.05.006. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaibazzi N., Martini C., Mattioli M., Tuttolomondo D., Guidorossi A., Suma S. Lung disease severity, coronary artery calcium, coronary inflammation and mortality in coronavirus disease 2019. Preprint at medRxiv. 2020 doi: 10.1101/2020.05.01.20087114. [DOI] [Google Scholar]
- 23.Giorgi Rossi P., Marino M., Formisano D., Venturelli F., Vicentini M., Grilli R. Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the Province of Reggio Emilia, Italy. Preprint at medRxiv. 2020 doi: 10.1101/2020.04.13.20063545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrillo-Vega M.F., Salinas-Escudero G., Garcia-Peña C., Gutierrez-Robledo L.M., Parra-Rodriguez L. Early estimation of the risk factors for hospitalisation and mortality by COVID-19 in Mexico. Preprint at medRxiv. 2020 doi: 10.1101/2020.05.11.20098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battisti S, Pedone C, Napoli N, Russo E, Agnoletti V, Nigra SG, et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19 [published online ahead of print, 2020 Aug 4]. Diabetes Care 2020: dc201333. 10.2337/dc20-1333. [DOI] [PubMed]
- 26.Chandarana H., Dane B., Mikheev A., Taffel M.T., Feng Y., Rusinek H. Visceral adipose tissue in patients with COVID-19: risk stratification for severity [published online ahead of print, 2020 Aug 3] Abdom Radiol (NY) 2020:1–8. doi: 10.1007/s00261-020-02693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamara A., Tahapary D.L. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr. 2020;14(4):655–659. doi: 10.1016/j.dsx.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik V.S., Ravindra K., Attri S.V., Bhadada S.K., Singh M. Higher body mass index is an important risk factor in COVID-19 patients: a systematic review and meta-analysis [published online ahead of print, 2020 Jul 24] Environ Sci Pollut Res Int. 2020:1–9. doi: 10.1007/s11356-020-10132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sales-Peres S.H.C., de Azevedo-Silva L.J., Bonato R.C.S., Sales-Peres M.C., Pinto A., Santiago Junior J.F. Coronavirus (SARS-CoV-2) and the risk of obesity for critically illness and ICU admitted: meta-analysis of the pidemiological evidence [published online ahead of print, 2020 Aug 3] Obes Res Clin Pract. 2020 doi: 10.1016/j.orcp.2020.07.007. S1871-403X(20)30555-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foldi M., Farkas N., Kiss S., Zadori N., Vancsa S., Szako L. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis [published online ahead of print, 2020 Jul 19] Obes Rev. 2020 doi: 10.1111/obr.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis [published online ahead of print, 2020 Jul 29] Diabetes Metab. 2020;S1262-3636(20):30097. doi: 10.1016/j.diabet.2020.07.005. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis [published online ahead of print, 2020 Jul 9]. Obes Res Clin Pract 2020; 14(4): 295–300. 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Retracted]
- 33.Yang J., Hu J., Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis [published online ahead of print, 2020 Jun 30] J Med Virol. 2020 doi: 10.1002/jmv.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soeroto A.Y., Soetedjo N.N., Purwiga A., Santoso P., Kulsum I.D., Suryadinata H. Association of BMI and obesity with composite poor outcome in COVID-19 adult patients: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.06.28.20142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettit NN, MacKenzie EL, Ridgway J, Pursell K, Ash D, Patel B, et al. Obesity is associated with increased risk for mortality among hospitalized patients with COVID-19 [published online ahead of print, 2020 Jun 26]. Obesity (Silver Spring) 2020. 10.1002/oby.22941. [DOI] [PMC free article] [PubMed]
- 36.Prevention CfDCa Adult obesity facts | overweight & obesity. 2020. https://www.cdc.gov/obesity/data/adult.html
- 37.World Health Organization Prevalence of obesity among adults, BMI ≥ 30, age-standardized - estimates by country. 2017. https://apps.who.int/gho/data/view.main.CTRY2450A?lang=en
- 38.Parri N., Lenge M., Buonsenso D. Coronavirus infection in pediatric emergency departments research G. children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383(2):187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. The Lancet. 2020;395(10236):1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50 [published online ahead of print, 2020 May 23] Obesity (Silver Spring) 2020 doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattar N., Ho F.K., Gill J.M., Ghouri N., Gray S.R., Celis-Morales C.A. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: preliminary findings from UK biobank. Diabetes Metab Syndr. 2020;14(5):1149–1151. doi: 10.1016/j.dsx.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Short K.R., Kedzierska K., van de Sandt C.E. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol. 2018;8:343. doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luzi L., Radaelli M.G. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57(6):759–764. doi: 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fezeu L., Julia C., Henegar A., Bitu J., Hu F.B., Grobbee D.E. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12(8):653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Kerkhove M.D., Vandemaele K.A., Shinde V., Jaramillo-Gutierrez G., Koukounari A., Donnelly C.A. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7) doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Littleton S.W. Impact of obesity on respiratory function. Respirology. 2012;17(1):43–49. doi: 10.1111/j.1440-1843.2011.02096.x. [DOI] [PubMed] [Google Scholar]
- 48.Shi L., Wang Y., Wang Y., Duan G., Yang H. Dyspnea rather than fever is a risk factor for predicting mortality in patients with COVID-19 [published online ahead of print, 2020 May 15] J Infect. 2020;S0163-4453(20):30288. doi: 10.1016/j.jinf.2020.05.013. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang J.F., Wang X.B., Zheng K.I., Liu W.Y., Chen J.J., George J. Letter to the editor: obesity hypoventilation syndrome and severe COVID-19. Metabolism. 2020;108:154249. doi: 10.1016/j.metabol.2020.154249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muscogiuri G., Pugliese G., Barrea L., Savastano S., Colao A. Commentary: obesity: the “Achilles heel” for COVID-19? Metabolism. 2020;108:154251. doi: 10.1016/j.metabol.2020.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y., Yang Q., Chi J., Dong B., Lv W., Shen L. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis [published online ahead of print, 2020 Jul 25] Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussain A., Vasas P., El-Hasani S. Letter to the editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154256. doi: 10.1016/j.metabol.2020.154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6) doi: 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maier H.E., Lopez R., Sanchez N., Ng S., Gresh L., Ojeda S. Obesity increases the duration of influenza a virus shedding in adults. J Infect Dis. 2018;218(9):1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan J., Grantham M., Pantelic J. Bueno de Mesquita PJ, Albert B, Liu F, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci U S A. 2018;115(5):1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korakas E., Ikonomidis I., Kousathana F., Balampanis K., Kountouri A., Raptis A. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020;319(1):E105. doi: 10.1152/ajpendo.00198.2020. E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sattar N., McInnes I.B., McMurray J.J.V. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 58.Chiappetta S., Sharma A.M., Bottino V., Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes (Lond) 2020;44(8):1790–1792. doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe M., Risi R., Tuccinardi D., Baquero C.J., Manfrini S., Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 2020:e3325. doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 60.West-Eberhard M.J. Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. Proc Natl Acad Sci U S A. 2019;116(3):723–731. doi: 10.1073/pnas.1809046116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu J., Yang S., Zhang A., Yang P., Cao X., Li X. Abdominal obesity is more closely associated with diabetic kidney disease than general obesity. Diabetes Care. 2016;39(10):e179–e180. doi: 10.2337/dc16-1025. [DOI] [PubMed] [Google Scholar]
- 62.Smith S.R., Lovejoy J.C., Greenway F., Ryan D. deJonge L, de la Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50(4):425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 63.Abraham T.M., Pedley A., Massaro J.M., Hoffmann U., Fox C.S. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639–1647. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neeland I.J., Ross R., Despres J.P., Matsuzawa Y., Yamashita S., Shai I. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 65.Petersen A., Bressem K., Albrecht J., Thiess H.M., Vahldiek J., Hamm B. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohkawara K., Tanaka S., Miyachi M., Ishikawa-Takata K., Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 2007;31(12):1786–1797. doi: 10.1038/sj.ijo.0803683. [DOI] [PubMed] [Google Scholar]
- 67.Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Jr., Liang L. Obesity & genetic predisposition with COVID-19. Metabolism. 2020;154345 doi: 10.1016/j.metabol.2020.154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A. Association of obesity with disease severity among patients with COVID-19. Obesity. 2020;28(7):1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G. Preprint at medRxiv. 2020. Characterization and clinical course of 1000 Patients with COVID-19 in New York: retrospective case series. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Sabah S.K., Al-Haddad M., Al Youha S., Jamal M.H., AlMazeedi S. COVID-19: impact of obesity and diabetes in disease severity. Preprint at medRxiv. 2020 doi: 10.1101/2020.05.24.20111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mejia-Vilet J.M., Cordova-Sanchez B.M., Fernandez-Camargo D., Mendez-Perez R.A., Morales-Buenrostro L.E., Hernandez-Gilsoul T. Derivation of a score to predict admission to intensive care unit in patients with COVID-19: the ABC-GOALS score. Preprint at medRxiv. 2020 doi: 10.1101/2020.05.12.20099416. [DOI] [PubMed] [Google Scholar]
- 74.Hur K., Price C.P.E., Gray E.L., Gulati R.K., Maksimoski M., Racette S.D. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163(1):170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M. Preprint at medRxiv. 2020. Determinants of severity in cancer patients with COVID-19 illness. [DOI] [Google Scholar]
- 76.Regina J., Papadimitriou-Olivgeris M., Burger R., Filippidis P., Tschopp J., Desgranges F. Preprint at medRxiv. 2020. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: an observational retrospective study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Antwi-Amoabeng D., Beutler B.D., Awad M., Kanji Z., Mahboob S., Ghuman J. Preprint at medRxiv. 2020. Sociodemographic predictors of outcomes in COVID-19: examining the impact of ethnic disparities in Northern Nevada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murillo-Zamora E., Hernandez-Suarez C.M. Survival in adult inpatients with COVID-19. Preprint at medRxiv. 2020 doi: 10.1101/2020.05.25.20110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halvatsiotis P., Kotanidou A., Tzannis K., Jahaj E., Magira E., Theodorakopoulou M. Demographic and clinical features of critically ill patients with COVID-19 in Greece: the burden of diabetes and obesity [published online ahead of print, 2020 Jul 17] Diabetes Res Clin Pract. 2020;166:108331. doi: 10.1016/j.diabres.2020.108331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rottoli M., Bernante P., Belvedere A., Balsamo F., Garelli S., Giannella M. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur J Endocrinol. 2020;183(4):389–397. doi: 10.1530/eje-20-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinberg E., Wright E., Kushner B. In young adults with COVID-19, obesity is associated with adverse outcomeS. West J Emerg Med. 2020;21(4):752–755. doi: 10.5811/westjem.2020.5.47972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakeshbandi M., Maini R., Daniel P., Rosengarten S., Parmar P., Wilson C. The impact of obesity on COVID-19 complications: a retrospective cohort study. Int J Obes (Lond) 2020:1–6. doi: 10.1038/s41366-020-0648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A-List of 59 disqualified papers
Appendix B-Table and reference list of three studies reporting VAT accumulation
Appendix C-Different effects between inclusion and exclusion of the study by Palaiodimos and co-authors
Appendix D-Table and forest plots of the three studies reporting SAT accumulation