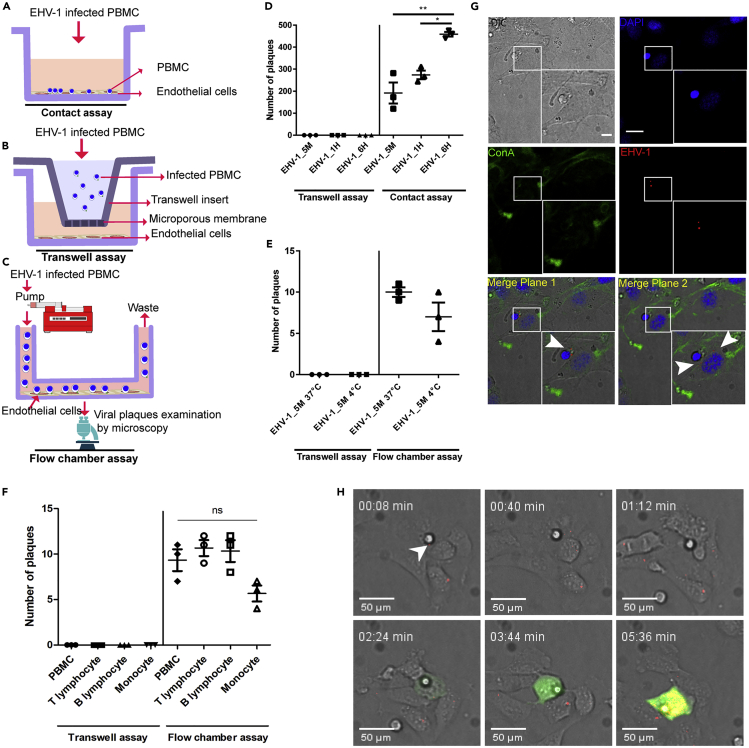
Figure 2.
Virus Transmission from Infected PBMC to EC under “Static” and “Dynamic” States
(A–F) Schematic depiction of contact (A), transwell (B), and flow chamber assays (C) is shown. PBMC were infected with EHV-1 GFP for different time periods (5min, 1 h, and 6 h). Infected PBMC were overlaid on EC under “static” conditions (D) or allowed to flow over EC “dynamic” (E and F) in the presence of neutralizing antibodies. Under dynamic conditions, whole PBMC population (E) and/or each PBMC subpopulation (T-lymphocyte, B-lymphocyte, monocyte); (F) were infected for 5 min either at 37°C (E and F) or at 4°C (E). After 24 h, virus spread was assessed by counting the plaques on EC excluding the inlet and outlet of the slide. As a control, infected PBMC or PBMC subpopulation were placed into a transwell insert without direct contact between EC and PBMC “no contact.” The data represent the mean ± standard deviation of three independent and blinded experiments. Significant differences in plaque numbers were seen between different infection points under “static” conditions (∗) (n = 3; one-way ANOVA test followed by multiple comparisons tests; ∗p < 0.05; ∗∗p < 0.01). PBMC in (F) refers to whole PBMC population.
(G) Confocal microscopy of overlaid infected PBMC on endothelial cells after 2 h. ECM was stained green with FITC-labeled ConA (green), EHV-1 RFP viral particles are red (arrowhead), and nucleus was stained with DAPI (blue). Data are representative of three independent experiments. Scale bar, 10 μm, and scale bar of magnification, 7 μm. Image stacks (number of stacks = 17 with 0.75 μm z stack step size) were photographed using VisiScope Confocal FRAP microscope. Presented here is a single optical section of the stacks.
(H) Confocal live-cell imaging showing the transfer of EHV-1 RFP + GFP viral particles (red; arrowhead) from overlaid 5-min-infected PBMC to the endothelial cells. Virus replication in EC is indicated by GFP expression (green), whereas RFP signals (at time points 03:44 and 05:36 min) represent new progeny RFP-labeled virus production (red) in the infected cells. Image stacks (number of stacks = 15 with 0.5 μm z stack step size) were photographed using VisiScope Confocal FRAP microscope. Presented here is a single optical section of the stacks.
See also Figures S6–S8.