Abstract
Endemic pemphigus foliaceus, known as Fogo Selvagem (FS) in Brazil, is a disease characterized by subcorneal blisters and IgG4 anti-Dsg1 autoantibodies. Epidemiological studies of FS strongly an environmental etiology. A 15 year surveillance of the Limao Verde Amerindian Reservation in Brazil has uncovered information on the transition of the autoimmune response from the pre-clinical stage to disease state. This incubation time may evolve over several years. The serological markers of pre-clinical state of FS are IgM anti-Dsg1, IgE and non-IgG4 autoantibodies against Desmoglein 1 (Dsg1). The disease stage of FS is characterized by the rise of pathogenic IgG4 anti-Dsg1 autoantibodies. In this review, the authors reviewed the literature on the relevance of the humoral autoimmune response of FS as well as the possible environmental triggers of anti-Dsg1 autoantibody formation. Based on epidemiological observations, the authors hypothesize that the pathogenic IgG4 response in FS may be triggered by hematophagous insect bites.
1. Non-endemic and endemic pemphigus foliaceus
The spectrum of clinical phenotypes of skin diseases known collectively as pemphigus include pemphigus vulgaris (PV), pemphigus foliaceus (PF), drug-induced pemphigus and paraneoplastic pemphigus (1). These diseases are characterized by spontaneous intraepidermal blister formation on the skin and mucous membranes and pathogenic autoantibodies against desmosomal glycoproteins (2–4). In PV and PF the autoantibodies recognize desmoglein 3 (Dsg3) and Dsg1 respectively (2). PF is a life-threatening skin disease characterized by subcorneal blisters and IgG anti-Dsg1 autoantibodies. These autoantibodies are known to be pathogenic by passive transfer experiments (4). Fogo Selvagem (FS) is an endemic form of PF which occurs among local inhabitants of rural Brazil (5), Colombia (6, 7) and Tunisia (8). FS shares similar clinical, histological and immunologic features with the non-endemic form of PF seen in the USA and around the world (9). Importantly, the IgG anti-Dsg1 autoantibodies are predominantly IgG4 and recognize the ectodomain of this molecule (10, 11).
We have described an active focus of FS in Brazil, the Limao Verde Amerindian Reservation, where the approximately 1,300 members of the Terena tribe show a ~3% prevalence of the disease (12). The following relevant observations have been made during the last 15 years of follow up of this human settlement: a) serological transition from preclinical to clinical stage of FS, including epitope mapping studies and IgG subclass switch; b) detection of IgM anti-Dsg1 autoantibodies in FS patients and healthy individuals from endemic areas; c) development of an IgG4-based classifier for FS sera, d) detection of anti-Dsg1 autoantibodies in the sera of patients with leishmaniasis, Chagas and onchocerciasis. Finally, we have detected a significant number of FS sera possessing IgE anti-Dsg1 autoantibodies that show correlation with IgG4 autoantibodies (13). These findings have prompted us to probe the immunological relationships of the IgG4 and IgE anti-Dsg1 autoantibody responses in FS patients and their possible link to a common environmental “allergen”, that in turn, may trigger the autoimmune response in FS.
2. The immune response against Dsg1 begins early in life with non-pathogenic IgM autoantibodies whereas the IgG response increases gradually with age.
Immunization of naïve individuals to a particular antigen is known to trigger a primary immune response characterized by low-affinity IgM antibodies. Re-exposure to the same antigen subsequently leads to a sustained secondary response characterized by high-affinity IgG antibodies (14). In humans, detection of antigen-specific IgM antibodies in sera may be an indicator of a recent infection or re-infection with viral diseases such as rubella (15), infectious mononucleosis (16) or hepatitis B (17), bacterial infections such as Lyme disease (18), or parasitosis such as toxoplasmosis (19, 20). We have found a relatively high prevalence of IgM anti-Dsg1 autoantibodies in sera from both FS patients and clinically normal donors residing in rural settings in or near an endemic area (21). Patients with PF, other autoimmune bullous diseases, as well as healthy individuals from more urban settings, did not demonstrate a significant IgM anti-Dsg1 response. A modest number of hospitalized FS patients that were temporarily isolated from their native outdoor environmental exposure showed positive IgM response. Importantly, over 50% of sera from healthy donors from three cohorts between the ages of 5 and 20 from Limao Verde possessed IgM anti-Dsg1 autoantibodies. There was no significant trend in IgM anti-Dsg1 autoantibody with age. A recent study showed that neonates from mothers with FS do not possess IgM anti-Dsg1 antibodies. Finally these studies showed that the prevalence of IgG anti-Dsg1 autoantibodies gradually increased with age of the donors (21). These novel findings support the hypothesis that an environmental antigen(s) sensitizes individuals living in these rural endemic areas of FS. This process began after the neonatal period of life because over 50% of children after the age of 5 already show IgM anti-Dsg1 autoantibodies in their sera. Further, they reinforce our findings that B cell repertoire in FS in the preclinical stage is antigen-driven.
While the observed seroepidemiology of the IgM anti-Dsg1 autoantibody response appears to suggest exposure to an environmental antigen(s), it is also known that unimmunized individuals may possess IgM antibodies to toxins, bacteria and erythrocytes that comprise a population of polyreactive low-affinity natural antibodies (22). These natural polyreactive IgM antibodies represent the first barrier against infection, eliminating bacteria by complement activation, thus bridging innate to adaptive immunity (22). Many of these IgM natural antibodies recognize single or multiple self-antigens (23) and have been detected from early childhood throughout life (24). Natural IgM autoantibodies against self-antigens have been reported in autoimmune diseases such as lupus erythematosus (25–27) autoimmune hemolytic anemia (28), and autoimmune thrombocytopenia (29). It is hypothesized that polyreactive IgM (27) or the IgG/IgM ratio of anti-dsDNA antibodies in systemic lupus erythematosus may modulate the disease and prognosis, especially in patients with nephritis (26).
In summary, these seroepidemiological observations suggest that the IgM response in FS patients from Limao Verde likely arises from recurrent and persistent antigenic exposure to an environmental cross-reactive antigen(s) harbored in this and other Amerindian reservations. Sensitization begins in early childhood and continues throughout life in these individuals, resulting in the production of non-pathogenic IgM and IgG anti-Dsg1 autoantibodies. Low affinity IgM anti-Dsg1 autoantibodies may not be demonstrated by routine indirect IF assays. Pathogenic IgG anti-Dsg1 autoantibodies and clinical disease may occur in only a small fraction of genetically predisposed individuals. Importantly, FS is more common among poor laborers of all races and both sexes sharing the HLA DRB1*0404, DRB1*1402 or DRB1*1406 alleles (RR: 14) (30). It is likely that serum concentrations of IgM anti-Dsg1 autoantibodies from FS patients referred to metropolitan hospitals, away from their native environment, decrease due to elimination of the environmental antigenic stimuli.
3. The pathogenic IgG response in FS is IgG4 restricted and predicts the disease. The incubation time of FS last years.
It has been known for several years that the humoral immune response against Dsg3 in PV is IgG4 restricted (31–39). This IgG4 restriction is also observed in non-endemic PF and FS. These studies have been confirmed using indirect IF, immunoblotting, and Dgs1 ELISA (36, 40–44). Moreover, we demonstrated that not only the total IgG4 was pathogenic but also its F(ab’)2 and Fab’ fragments when tested in the mouse model of FS (45). Additional studies in FS have demonstrated that the autoantibody response in FS exhibits a limited heterogeneity, consisting of oligoclonal IgG1 and IgG4 banding, when tested with epidermal antigens by affinity immunoblotting (46). These studies suggested that the autoantibody response in FS exhibits an early IgG1 response followed by a sustained IgG4 response.
In a recent study we conducted the serological evaluation of IgG and IgG subclass anti-Dsg1 autoantibodies in 214 FS cases (45%) and 261 normal individuals (55%) and generated a highly sensitive and specific “IgG4 classifier/ predictor” (47). FS patients were seen in 5 Brazilian hospitals (Campo Grande, Sao Paulo, Goiania, Brasilia and Belho Horizonte) from 1980 to 2006. Healthy controls were obtained from the blood banks of the same Brazilian hospitals and from UNC Hospital. The sera were tested by Dsg1 ELISA optimized for the detection of human IgG subclasses using murine monoclonal anti-human IgG subclass-specific antibodies and the results were expressed as index values. A logistic regression model was used to develop a “classifier” that predicts case-control status based on the four IgG subclass index values. For the purpose of developing and evaluating a classification rule, the data set (n= 475 samples) was divided at random into three parts; a training set (n=239), a validation set (n=118) and a test set (n=118) containing 50%, 25% and 25% of the cases and controls, respectively. A rigorous statistical procedure was applied in order to choose the best model for prediction. Each of 15 possible models were estimated from the training set. Sensitivity, specificity and area under the curve (AUC) were estimated from the validation set. The AUC is a summary of the whole ROC curve for a given model and is not affected by choice of the cut point. Thus AUC was used as the criterion for model selection. An observation with fitted probability above 0.45 was classified as a case. The model with only IgG4 had an AUC of 0.961 (in the validation set). Using additional predictors had a negligible effect on the AUC. Thus, the model with only IgG4 was chosen as the final model because of its parsimony and high AUC value.
The classification rule based on IgG4 classify a given subject as a case if the IgG4 index value exceeds 6.43, otherwise classify as a non-case. The estimated AUC is 0.971 (95% CI: 94–100%), sensitivity is 92% (95% CI: 82–98%), and specificity is 97% (95% CI: 89–100%) of the “classifier” was determined on the test set. It is worth mentioning that the “classifier” was developed entirely in the training and validation sets, yet it performed extremely well in the test set.
The positive predictive value (PPV) and negative predictive value (NPV) of the classifier, when applied in a population such as the Limao Verde reservation where the prevalence of FS is 3% were calculated. It was estimated that an individual from Limao Verde, classified as positive by the classifier, has a 49% chance of having FS while a subject classified as negative has a 99.7% probability of being disease-free. The PPV and NPV for IgG and other IgG subclasses were lower than IgG4. Since the prevalence of IgG anti-Dsg1 autoantibodies in normal inhabitants of endemic areas of FS, i.e. Limao Verde is high (21, 48), the use of IgG as a classifier in these human settlements would be very limited. On the contrary, IgG4 anti-Dsg1 autoantibodies are detected in the sera of individuals developing FS or during recurrences (49, 50). For similar reasons the IgM anti-Dsg1 autoantibodies (21) did not performed well.
The IgG4-based classifier was validated further by analyzing other groups of patients and people at risk to develop FS:
a). Eleven FS patients during the preclinical stage of the disease.
In the first group, the classifier predicted FS in 5/11 individuals (45%) during the preclinical stage and in all samples during the clinical stage of FS (100%). It must be emphasized that this classifier identifies subjects with serological features of FS regardless of the presence of active skin disease. We propose that this IgG4-based classifier is a serological marker of FS during the preclinical and clinical stages of the disease. During the preclinical stage this classifier may show variations over time due to fluctuations in environmental antigenic stimulation.
b). Sixty Japanese patients.
Twenty patients with mucosal PV, possessing only anti-Dsg3 autoantibodies, were classified as normal donors by the classifier (because the absence of anti-Dsg1 autoantibodies). The IgG4-based classifier performed well in a group of 20 PF patients, of whom 18 were identified as cases. In a group of 20 mucocutaneous PV patients, possessing anti-Dsg1 and anti-Dsg3 autoantibodies, the classifier predicted the disease in 17 cases. Hence the IgG4-based classifier performed well in the Japanese group of patients with PF and mcPV since both groups of patients possess anti-Dsg1 autoantibodies.
c). Three cohorts (n=96) of normal individual (ages 5–20) from Limao Verde.
The IgG4 classifier identified 21 individuals (22%) with serological features of FS: 6/34 individuals (17.6%) in cohort 1 (age 5–10) were positive, 6/39 (15.3%) in cohort 2 (age 11–15) were also positive as well as 9/24 (37.5%) in cohort 3 (age 16–20). The same donors show the following percentages of total IgG anti-Dsg1 autoantibodies: cohort 1, 1/34 (2.9%), cohort 2, 3/41 (7.3%) and cohort 3, 7/24 (29%). Interestingly, one member of the third cohort (JDM), classified as a case, has developed FS in the course of the study. According to the PPV of the IgG4 classifier, it is estimated that about 50% of these positive subjects from Limao Verde have FS in the preclinical stage and are at risk to develop clinical disease if the conditions are appropriate. Similarly, each of the 75 subjects identified as normal by the classifier have a 99% chance of being disease-free. Forecasting active clinical disease in individuals of both groups (positive and negative) using the classifier is the subject of current investigation in our laboratory. An ongoing prospective study of these cohorts will validate further this immunological instrument not only as an identifier of current FS serology but also as a predictor of future disease. These cohorts are evaluated clinically every 4 months and serologically every 2 years.
It is concluded from these studies that the bulk of pathogenic anti-Dsg1 autoantibodies in FS are predominantly IgG4 (40, 50). In fact, a recent study showed that progression from preclinical to clinical stage of the disease is associated with a dramatic rise in IgG4 anti-Dsg1 autoantibodies as determined by ELISA assays (50). Further,, we believe that the IgG4 anti-Dsg1 based classifier would be extremely useful in identifying individuals during the preclinical stage of FS. HLA typing and the IgG4-based classifier would become powerful tools for the selection of individuals to undergo close clinical and serological surveillance. Moreover, as the environmental risk factor(s) can also be assessed among potential FS patients, these immunological markers may enhance our ability to identify these factor(s) involved in triggering the autoimmune disease in FS.
4. Antigen selection of anti-Dsg1 autoantibody occurs not only during but also prior to the onset of FS disease:
The diversity and clonality of the anti-Dsg1 response, as well as the likelihood that anti-Dsg1 B cells are antigen selected, were studied with peripheral blood samples from 8 FS patients and one individual with prior-to-clinical FS (51). Human hybridomas were generated by fusion of EBV-transformed blood lymphocytes with either mouse myeloma cells (P3×63Ag8.653) or MFP-2s (52) myeloma cells as fusion partners. Dsg1 was used to detect anti-Dsg1 antibodies by ELISA during the screening and cloning of hybridomas. The V genes of both H and L chains of the Dsg-specific autoantibodies from these hybridomas were sequenced and analyzed for the H and L chain pairing of the autoantibodies, the diversity of H and L chain V gene usage, and the extent of somatic mutation. Seventy-eight monoclonal anti-Dsg1 hybridomas were isolated; 38 secreted IgG, and 40 secreted IgM. Multiple lines of evidence suggest that the anti-Dsg1 autoimmune response in FS is antigen selected. First, clonally related sets of anti-Dsg1 hybridomas were identified from individual FS patients. Second, H and L chain V gene use appears to be biased, particularly among IgG hybridomas, as some H chain V gene families were over-represented and others under-represented. Third, both IgM and IgG hybridomas exhibited a high frequency of VH mutation. The replacement versus silent mutation (R/S) ratios exhibit a bias in favor of CDR (complementary determining region) amino acid replacement mutation and in favor of silent mutation of FWRs (framework regions). Interestingly, similar selection was evident among anti-Dsg1 hybridomas from an individual with prior-to-clinical disease. The V genes of hybridomas from this individual were also extensively mutated, and showed similar R/S mutation bias in CDRs and FWRs of their H and L chain V genes with that of FS patients. Thus, selection of anti-Dsg1 B cells begins well before the onset of the FS disease. This is consistent with the hypothesis that an environmental antigen(s) may induce the cross-reactive anti-Dsg1 response in these genetically predisposed individuals to initiate the FS.
5. Fogo Selvagem is an environmentally triggered autoimmune disease of the skin.
FS, shows several unique and remarkable features such as the geographic and temporal clustering of cases, the increased frequency of cases among young adults and children, the increased frequency of familial cases, and an association with certain distinct HLA-DR alleles (30). FS occurs in Brazilian states located between 45o to 60o west longitude and 5o to 25o south latitude in regions with an altitude between 500–800 meters (1,600–2,600 feet). FS is rare at altitudes below 400 meters (1,300 feet) or above 1,000 meters (3,300 feet) (53, 54). The weather in endemic regions of FS is subtropical and in northern regions supports coffee, sugarcane and cacao, while in southern regions corn, soybeans and cotton are the predominant crops.
The FS patient is usually a farmer (or a member of the family) who farms in these fields or works outdoors. They are usually young adults or children (5). In general, poor families move to a farm and live in rustic houses located near rivers or creeks. It is also common among them to raise chickens, hogs, cows, horses and have a variety of pets such as dogs, cats, and birds. There is no reported sex or race predisposition for the development of FS (53–56). The daily activities of a family include agriculture, care of livestock and home chores. Wives and children remain at home to perform routine chores, i.e. cooking, caring for small animals or washing laundry in nearby rivers or streams. However, a great number of wives help their husbands in farming activities. The farmers and his family usually have a common bedroom and wear light clothes appropriate for the weather. The houses are usually built of reed walls and thatched roofs with open doors and open windows. Commonly, these houses harbor rodents, other small wild animals and are usually infested with blood-feeding arthropods such as bedbugs and Reduvid bugs. Because they contain shallow waters and rock beds, some rivers and streams are infested with a variety of insects, including Simuliids (black fly, also known as “borrachudo” in Portuguese) and sandflies. The number of new cases of FS is greatest at the end of the rainy season (September to March) and least during the dry summer (April to August), suggesting that insect multiplication and increase in the number of FS patients are related phenomena (53, 54, 56). Deforestation and the arrival of humans to settle in these regions has been a salient feature of FS as described by Brazilian investigators.
Interestingly, the same ecological systems found in the “pemphigus country” overlap with those described in Chagas disease (57–59), Leishmaniasis (60–62) and Onchocerciasis (63, 64). These diseases, as well as FS, were initially reported in Brazil during the first decade of the 20th century affecting the newly arrived pioneers to previously unconquered lands (57, 62, 65). The diseases were common among workers laboring in the construction of railroads and highways in the interior of the endemic states, i.e. Sao Paulo, Minas Gerais. It is known that these diseases tend to disappear once the working and living conditions of the settlers improve. In recent years the frequency of cases of Chagas disease, Leishmaniasis and FS in the endemic states, as compared with those seen in the middle part of the 20th century has dramatically decreased (66–72) and this decrease has been associated with modernization in agricultural techniques (67). It is clear, that the ecological systems of FS share similarities with these insect vector borne diseases.
Finally, the Amerindian reservation of Limao Verde, located in the state of Mato Grosso do Sul, Brazil, is the home of 1,351 members of the Terena tribe of Amerindians (as of September 10, 2006), and is an active focus of FS, exhibiting a ~3 % prevalence of disease (12, 48). The people of this reservation are heavily exposed to hematophagous insects inside and outside of their homes (73) and these insects are considered risk factors for FS (74). For the last 15 years, we have periodically collected clinical and serological data from FS patients as well as clinically normal individuals residing in and around this settlement. We have found that the autoantibody response against Dsg1 in healthy individuals from Limao Verde and neighboring communities is common, and directly related to physical distance to this reservation (21, 48, 75). Over the course of this investigation, we have also observed the clinical and serological conversion from normal to disease state in eleven individuals (47–49).
6. Possible etiological factors of Fogo Selvagem-Hypothesis.
The diagram shown in Figure 1 depicts the central hypothesis investigated in our laboratory about the immunological events triggered in genetically predisposed individuals that lead to FS. As described above, and based on the information generated from the Limao Verde endemic focus of FS, we hypothesize that exposure of this population to an environmental risk factor triggers the cross-reactive autoimmune response that leads to FS. During early childhood a substantial number of individuals start producing IgM anti-Dsg1 autoantibodies, a process that may continues for years if the person remains in Limao Verde. It is feasible, although unproven, that in individuals moving out of an endemic area such as Limao Verde, this IgM anti-Dsg1 response may decrease and disappear. The IgG response in the Limao Verde population appears after the age of 5 years and continues through adulthood. The IgG anti-Dsg1 autoantibodies may be found in up to 1/3 of the normal population and have no pathogenic consequences in the individual. Our studies demonstrate that, when the IgG anti-Dsg1 response in normal individuals shows a predominant IgG4 autoantibodies, this individual will be at high risk to develop FS. The IgM and IgG response against Dsg1 remain as a serological marker of the preclinical stage of FS. After an incubation time that may last from 1 to more than 10 years, some genetically predisposed subjects may increase their IgG4 anti-Dsg1 autoantbodies titers and develop clinical FS.
Figure 1:
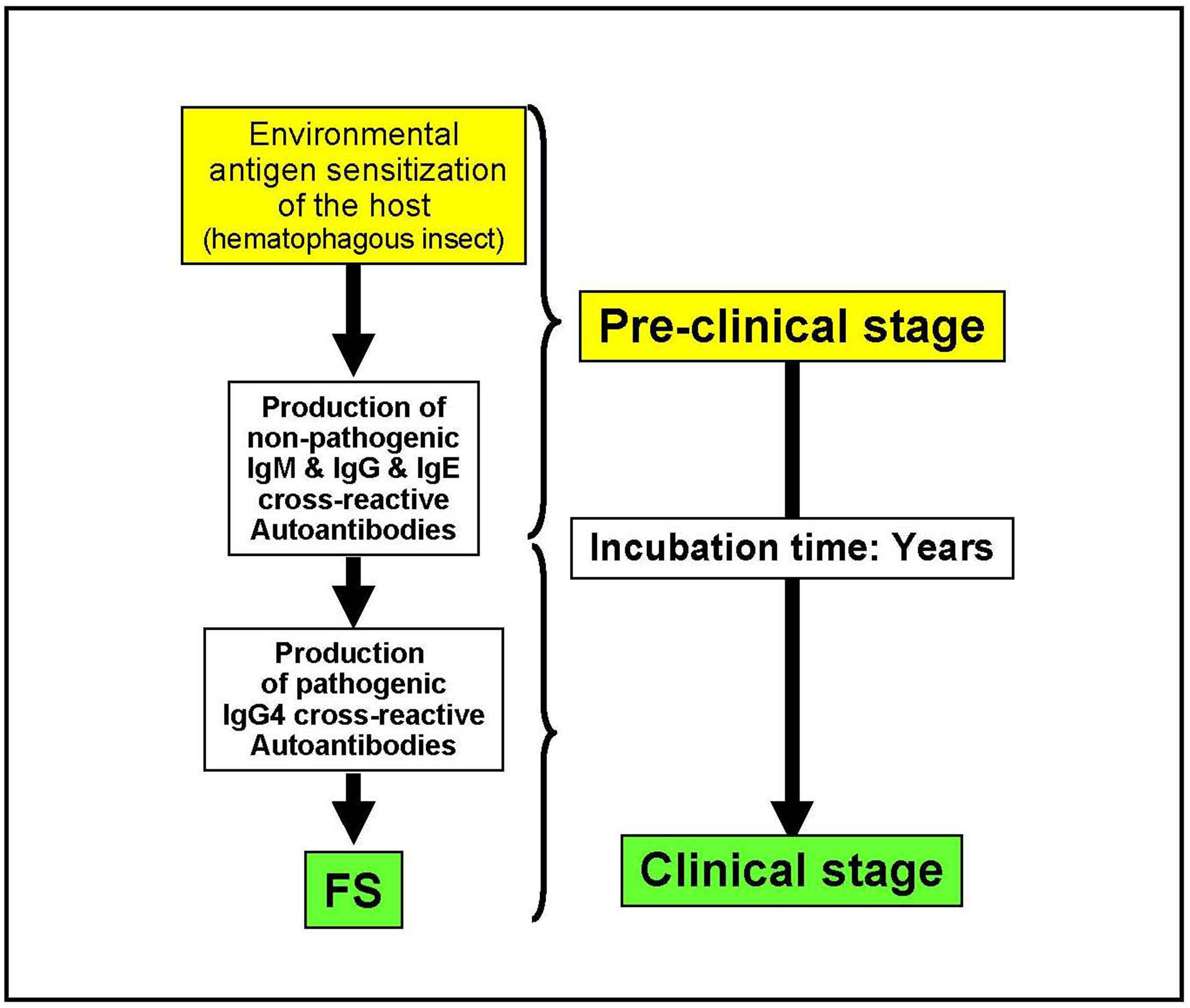
Pathogenesis of Fogo Selvagem: Hypothesis
The biochemical interactions between a vector and a host’s skin during the process of blood feeding by hematophagous insects is complex (76),. In order to steal a blood meal from the host, the hematophagous vector must move quickly using mechanical tools (mouthparts) to perforate the skin and inject a cocktail of salivary substances to counteract host defense mechanisms (i.e coagulation, vasoconstriction, platelet aggregation, etc) (77–80). The host defense ranges from the mechanical squashing of the offending insect to the in situ tissue and humoral response at the site of the bite, i.e vasoconstriction, inflammation and antibody response. Medically relevant Insects (blood feeding and others) trigger several responses in the host: (i) an acute or chronic process in which the host may exhibits a local or systemic reaction modulated by IgE and IgG4 antibodies against an insect product (e.g. bee venom) (81); (ii) they are carriers of disease causing parasites (e.g. hematophagous bugs in leishmaniasis, Chagas disease, filariasis, malaria, etc.); (iii) sensitization and production of anti-saliva antibodies that block parasite invasion as occurs in malaria or leishmaniasis (82) where salivary proteins may promote parasite invasion (77–80). Additionally, in filariasis there is a predominantly IgG4 response (83). Interestingly, a small number of FS patients have antibodies to maxadilan, a potent vasodilator isolated from sand fly saliva (84). Although some of the salivary components from blood-feeding insects have been extensively characterized at the molecular level (85–89), others including cross-reactive carbohydrate determinants (90, 91) remain unstudied.
We are testing the intriguing hypothesis that a component(s) of the saliva of these hematophagous insects (reduvii, simuliid and lutzomia) contain cross-reactive antigens with human epidermal Dsg1 and thus triggers the anti-Dsg1 autoantibody response (Figure 2). Interestingly, the genome of a well-characterized insect like the fruit fly Drosophila melanogaster, contains 19 genes encoding cadherin-like molecules (92); whether these molecules are present in the saliva of these insects is unknown. Considering the persistent IgM anti-Dsg1 response in normal individuals of Limao Verde we entertain the idea that the sensitizing antigen(s) may be a T-independent (TI) type 2 antigen, perhaps carbohydrate, derived from the environment (blood feeding bugs, parasites etc) (93, 94). Examples of TI-2 antigens include among other, multivalent polysaccharides (or other antigens with repetitive structures) from Streptococcus pneumonia, Haemophilus influenza and Neisseria meningitides (93–95). Interestingly, Nedelect et al (96) showed that capsular polysaccharide from Neisseria meningitides shares epitopes and molecularly mimics the neural cell adhesion molecule (NCAM) and that IgM antibodies produced by patients may be cytotoxic.
Figure 2:
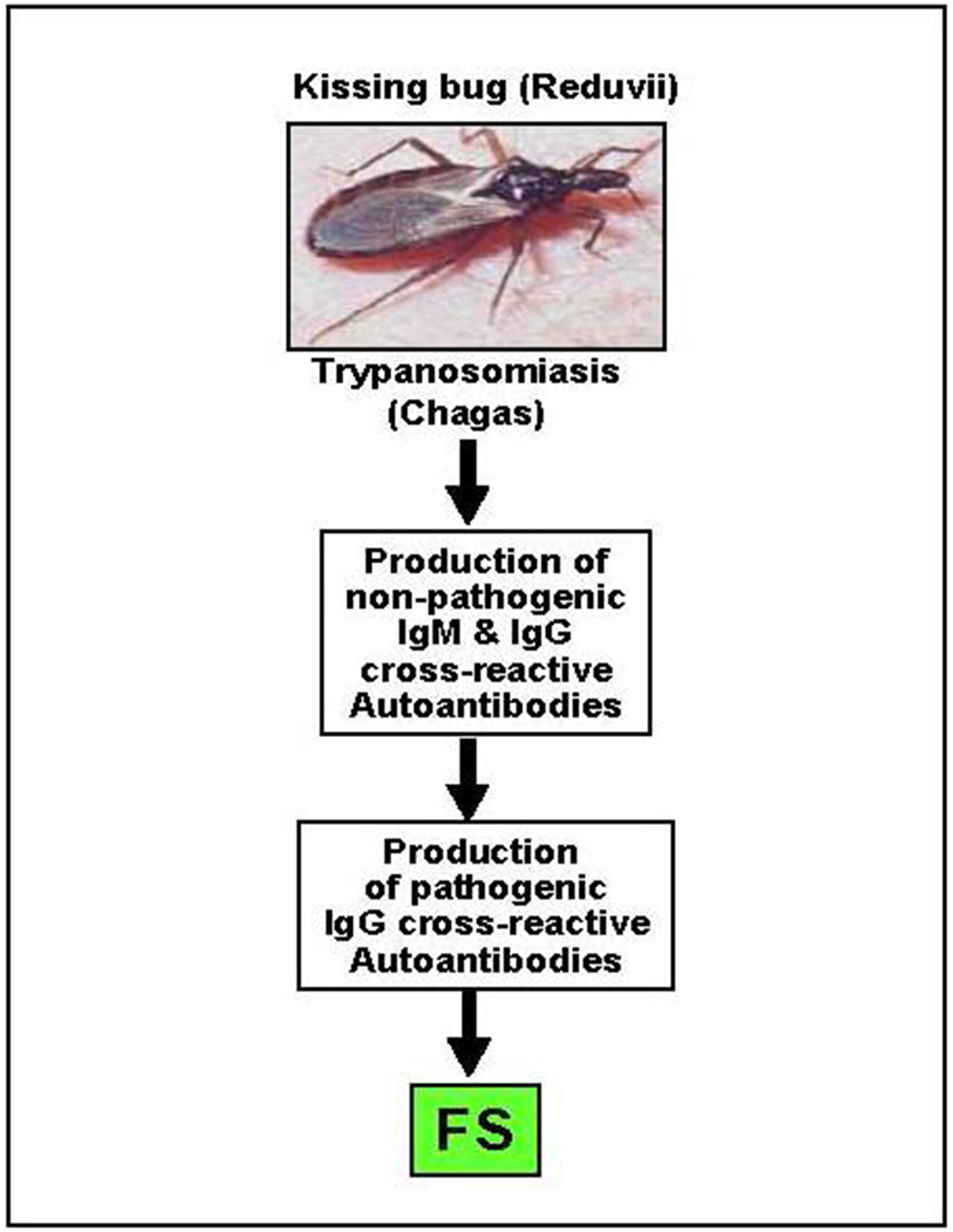
Hematophagous insects and Fogo Selvagem
Acknowledgements:
This work was supported in part by U.S. Public Health Service Grants R01AR30281, R01AR32599 awarded to Dr. Diaz and K01AR056378 to Dr. Qian. Dr Qian’s research is also supported by a Dermatology Foundation Research Grant and an American Skin Association Alice P. Melly Research Grant. Dr. Gustavo Flores is a Dermatology Research Fellow.
Abbreviations:
- FS
fogo selvagem
- Dsg1
desmoglein 1
- Dsg3
desmoglein 3
- PF
pemphigus foliaceus
- PV
pemphigus vulgaris
- ELISA
enzyme-linked immunosorbent assay
References
- 1.Bystryn JC, Rudolph JL. Pemphigus. Lancet. 2005;366:61–73. [DOI] [PubMed] [Google Scholar]
- 2.Udey MC, Stanley JR. Pemphigus--diseases of antidesmosomal autoimmunity. JAMA. 1999;282:572–6. [DOI] [PubMed] [Google Scholar]
- 3.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–96. [DOI] [PubMed] [Google Scholar]
- 4.Roscoe J, Diaz L, Sampaio S, Castro R, Labib R, Takahashi Y, et al. Brazilian Pemphigus Foliaceus Autoantibodies Are Pathogenic to BALB/c Mice by Passive Transfer. J Invest Dermatol. 1985;85:538–41. [DOI] [PubMed] [Google Scholar]
- 5.Diaz LA, Sampaio SA, Rivitti EA, Martins CR, Cunha PR, Lombardi C, et al. Endemic pemphigus foliaceus (Fogo Selvagem): II. Current and historic epidemiologic studies. J Invest Dermatol. 1989;92:4–12. [DOI] [PubMed] [Google Scholar]
- 6.Abreu-Velez AM, Hashimoto T, Bollag WB, Tobon Arroyave S, Abreu-Velez CE, Londono ML, et al. A unique form of endemic pemphigus in northern Colombia. J Am Acad Dermatol. 2003;49:599–608. [DOI] [PubMed] [Google Scholar]
- 7.Robledo MA, Prada S, Jaramillo D, Leon W. South American pemphigus foliaceus: study of an epidemic in El Bagre and Nechi, Colombia 1982 to 1986. Br J Dermatol. 1988;118:737–44. [DOI] [PubMed] [Google Scholar]
- 8.Morini JP, Jomaa B, Gorgi Y, Saguem MH, Nouira R, Roujeau JC, et al. Pemphigus foliaceus in young women. An endemic focus in the Sousse area of Tunisia. Arch Dermatol. 1993;129:69–73. [DOI] [PubMed] [Google Scholar]
- 9.Diaz L, Sampaio S, Rivitti E, Martins C, Cunha P, Lombardi C, et al. Endemic pemphigus foliaceus (fogo selvagem). I. Clinical features and immunopathology. J Am Acad Dermatol. 1989;20:657–69. [DOI] [PubMed] [Google Scholar]
- 10.Emery DJ, Diaz LA, Fairley JA, Lopez A, Taylor AF, Giudice GJ. Pemphigus foliaceus and pemphigus vulgaris autoantibodies react with the extracellular domain of desmoglein-1. J Invest Dermatol. 1995;104:323–8. [DOI] [PubMed] [Google Scholar]
- 11.Futamura S, Martins C, Rivitti EA, Labib RS, Diaz LA, Anhalt GJ. Ultrastructural studies of acantholysis induced in vivo by passive transfer of IgG from endemic pemphigus foliaceus (Fogo Selvagem). J Invest Dermatol. 1989;93:480–5. [DOI] [PubMed] [Google Scholar]
- 12.Hans-Filho G, dos Santos V, Katayama JH, Aoki V, Rivitti EA, Sampaio SA, et al. An active focus of high prevalence of fogo selvagem on an Amerindian reservation in Brazil. Cooperative Group on Fogo Selvagem Research. J Invest Dermatol. 1996;107:68–75. [DOI] [PubMed] [Google Scholar]
- 13.Qian Y, Qaqish BF, Andraca E, Flores G, Aoki V, Hans-Filho G, et al. IgE anti-desmoglein 1 autoantibodies and its implication on the etiology of endemic pemphigus foliaceus. 2009.
- 14.Abbas AK, Lichtman AH, Pober JS. B cell activation and antibody production Cellular and molecular immunology. 5th Ed-Updated. ed Philadelphia, PA: Elsevier Saunders, 2005. p. 190–1. [Google Scholar]
- 15.Hamkar R, Jalilvand S, Mokhtari-Azad T, Nouri Jelyani K, Dahi-Far H, Soleimanjahi H, et al. Assessment of IgM enzyme immunoassay and IgG avidity assay for distinguishing between primary and secondary immune response to rubella vaccine. J Virol Methods. 2005;130:59–65. [DOI] [PubMed] [Google Scholar]
- 16.Obel N, Hoier-Madsen M, Kangro H. Serological and clinical findings in patients with serological evidence of reactivated Epstein-Barr virus infection. APMIS. 1996;104:424–8. [DOI] [PubMed] [Google Scholar]
- 17.Chu CM. Immunoglobulin class M anti-hepatitis B core antigen for serodiagnosis of acute hepatitis: pitfalls and recommendations. J Gastroenterol Hepatol. 2006;21:789–91. [DOI] [PubMed] [Google Scholar]
- 18.Cermakova Z, Ryskova O, Honegr K, Cermakova E, Hanovcova I. Diagnosis of Lyme borreliosis using enzyme immunoanalysis. Med Sci Monit. 2005;11:BR121–5. [PubMed] [Google Scholar]
- 19.Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis. 2002;185 Suppl 1:S73–82. [DOI] [PubMed] [Google Scholar]
- 20.Reis MM, Tessaro MM, D’Azevedo PA. Toxoplasma-IgM and IgG-avidity in single samples from areas with a high infection rate can determine the risk of mother-to-child transmission. Rev Inst Med Trop Sao Paulo. 2006;48:93–8. [DOI] [PubMed] [Google Scholar]
- 21.Diaz LA, Prisayanh PS, Dasher DA, Li N, Evangelista F, Aoki V, et al. The IgM anti-desmoglein 1 response distinguishes Brazilian pemphigus foliaceus (fogo selvagem) from other forms of pemphigus. J Invest Dermatol. 2008;128:667–75. [DOI] [PubMed] [Google Scholar]
- 22.Manson JJ, Mauri C, Ehrenstein MR. Natural serum IgM maintains immunological homeostasis and prevents autoimmunity. Springer Semin Immunopathol. 2005;26:425–32. [DOI] [PubMed] [Google Scholar]
- 23.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–8. [DOI] [PubMed] [Google Scholar]
- 24.Mouthon L, Lacroix-Desmazes S, Nobrega A, Barreau C, Coutinho A, Kazatchkine MD. The self-reactive antibody repertoire of normal human serum IgM is acquired in early childhood and remains conserved throughout life. Scand J Immunol. 1996;44:243–51. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira R, Barreto M, Santos E, Pereira C, Martins B, Andreia R, et al. Heritable factors shape natural human IgM reactivity to Ro60/SS-A and may predispose for SLE-associated IgG anti-Ro and anti-La autoantibody production. J Autoimmun. 2005;25:155–63. [DOI] [PubMed] [Google Scholar]
- 26.Forger F, Matthias T, Oppermann M, Becker H, Helmke K. Clinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritis. Lupus. 2004;13:36–44. [DOI] [PubMed] [Google Scholar]
- 27.Li QZ, Xie C, Wu T, Mackay M, Aranow C, Putterman C, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahl D, Hoemberg M, Cassens U, Pachmann U, Sibrowski W. Evidence that human autoimmune thrombocytopenia mediated by both immunoglobulin isotypes IgM and IgG is an independent disease entity. Eur J Haematol. 2005;75:318–27. [DOI] [PubMed] [Google Scholar]
- 29.Stahl D, Sibrowski W. Warm autoimmune hemolytic anemia is an IgM-IgG immune complex disease. J Autoimmun. 2005;25:272–82. [DOI] [PubMed] [Google Scholar]
- 30.Moraes M, Fernandez-Vina M, Lazaro A, Diaz Filho L, Friedman H, Rivitti E, et al. An epitope in the third hypervariable region of the DRB1 gene is involved in the susceptibility to endemic pemphigus foliaceus (fogo selvagem) in three different Brazilian populations. Tissue Antigens. 1997;49:35–40. [DOI] [PubMed] [Google Scholar]
- 31.Bhol K, Natarajan K, Nagarwalla N, Mohimen A, Aoki V, Ahmed A. Correlation of Peptide Specificity and IgG Subclass with Pathogenic and Nonpathogenic Autoantibodies in Pemphigus Vulgaris: A Model for Autoimmunity. Proc Natl Acad Sci U S A. 1995;92:5239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks W, Lee Y, Abell E, Deng J. Comparison of IgG subclasses and complement binding activity of autoantibodies from patients with bullous pemphigoid and pemphigus. J Clin Lab Anal. 1989;3:307–11. [DOI] [PubMed] [Google Scholar]
- 33.David M, Katzenelson V, Hazaz B, Ben-Chetrit A, Sandbank M. Determination of IgG subclasses in patients with pemphigus with active disease and in remission. Arch Dermatol. 1989;125:787–90. [PubMed] [Google Scholar]
- 34.Jones CC, Hamilton RG, Jordon RE. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988;8:43–9. [DOI] [PubMed] [Google Scholar]
- 35.Parlowsky T, Welzel J, Amagai M, Zillikens D, Wygold T. Neonatal pemphigus vulgaris: IgG4 autoantibodies to desmoglein 3 induce skin blisters in newborns. J Am Acad Dermatol. 2003;48:623–5. [DOI] [PubMed] [Google Scholar]
- 36.Hacker MK, Janson M, Fairley JA, Lin MS. Isotypes and antigenic profiles of pemphigus foliaceus and pemphigus vulgaris autoantibodies. Clin Immunol. 2002;105:64–74. [DOI] [PubMed] [Google Scholar]
- 37.Torzecka JD, Wozniak K, Kowalewski C, Waszczykowska E, Sysa-Jedrzejowska A, Pas HH, et al. Circulating pemphigus autoantibodies in healthy relatives of pemphigus patients: coincidental phenomenon with a risk of disease development? Arch Dermatol Res. 2007;299:239–43. [DOI] [PubMed] [Google Scholar]
- 38.Yamada H, Hashimoto T, Nishikawa T. IgG Subclasses of Intercellular and Basement Membrane Zone Antibodies: The Relationship to the Capability of Complement Fixation. J Invest Dermatol. 1989;92:585–7. [DOI] [PubMed] [Google Scholar]
- 39.Yeh SW, Cavacini LA, Bhol KC, Lin MS, Kumar M, Duval M, et al. Pathogenic human monoclonal antibody against desmoglein 3. Clin Immunol. 2006;120:68–75. [DOI] [PubMed] [Google Scholar]
- 40.Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem). N Engl J Med. 1989;320:1463–9. [DOI] [PubMed] [Google Scholar]
- 41.Allen EM, Giudice GJ, Diaz LA. Subclass reactivity of pemphigus foliaceus autoantibodies with recombinant human desmoglein. J Invest Dermatol. 1993;100:685–91. [DOI] [PubMed] [Google Scholar]
- 42.Marques Babosa Dos Santos S, Lima Filgueira A, Andrade Patrus O, Diaz L. Perfil evolutivo das subclasses de imunoglobulinas gama em pacientes de pênfigo foliáceo endêmico. Anais brasileiros de dermatologia. 2001;76:561–74. [Google Scholar]
- 43.Hacker-Foegen MK, Janson M, Amagai M, Fairley JA, Lin MS. Pathogenicity and epitope characteristics of anti-desmoglein-1 from pemphigus foliaceus patients expressing only IgG1 autoantibodies. J Invest Dermatol. 2003;121:1373–8. [DOI] [PubMed] [Google Scholar]
- 44.Aoki V, Huang MH, Perigo AM, Fukumori LM, Maruta CW, Santi CG, et al. Endemic pemphigus foliaceus (fogo selvagem) and pemphigus vulgaris: immunoglobulin G heterogeneity detected by indirect immunofluorescence. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:251–6. [DOI] [PubMed] [Google Scholar]
- 45.Rock B, Labib R, Diaz L. Monovalent Fab’immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. J Clin Invest. 1990;85:296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calvanico N, Swartz S, Diaz L. Affinity immunoblotting studies on the restriction of autoantibodies from endemic pemphigus foliaceus patients. J Autoimmun. 1993;6:145–57. [DOI] [PubMed] [Google Scholar]
- 47.Qaqish BF, Prisayanh P, Qian Y, Andraca E, Li N, Aoki V, et al. Development of an IgG4-based predictor of endemic pemphigus foliaceus (fogo selvagem). J Invest Dermatol. 2009;129:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren SJ, Lin MS, Giudice GJ, Hoffmann RG, Hans-Filho G, Aoki V, et al. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. Cooperative Group on Fogo Selvagem Research. N Engl J Med. 2000;343:23–30. [DOI] [PubMed] [Google Scholar]
- 49.Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem). J Exp Med. 2003;197:1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren S, Arteaga L, Rivitti E, Aoki V, Hans-Filho G, Qaqish B, et al. The Role of Subclass Switching in the Pathogenesis of Endemic Pemphigus Foliaceus. J Invest Dermatol. 2003;120:104–8. [DOI] [PubMed] [Google Scholar]
- 51.Qian Y, Clarke SH, Aoki V, Hans-Filhio G, Rivitti EA, Diaz LA. Antigen Selection of Anti-DSG1 Autoantibodies During and Before the Onset of Endemic Pemphigus Foliaceus. J Invest Dermatol. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalantarov GF, Rudchenko SA, Lobel L, Trakht I. Development of a fusion partner cell line for efficient production of human monoclonal antibodies from peripheral blood lymphocytes. Hum Antibodies. 2002;11:85–96. [PubMed] [Google Scholar]
- 53.Aranha Campos J. Penfigo foliaceo (Fogo Selvagem). Aspectos clinicos e epidemiologicos. Comp. Melhoramentos S. Paulo, 1942. [Google Scholar]
- 54.Auad A. Penfigo foliaceo Sul-Americano no Estado de Goias. Rev Patol Trop. 1972;1:293–346. [Google Scholar]
- 55.Minelli L. Geografia Medica do Penfigo Foliaceo Sul Americano no Estado do Parana. An Bras Dermatol Sifilol. 1976;51:173–81. [Google Scholar]
- 56.Vieira J. Pemphigus Foliaceus (Fogo Selvagem). An Endemic Disease of the State of Sao Paulo (Brazil). Arch Dermatol. 1940;41:858–63. [Google Scholar]
- 57.Dias J, Schofield C. The evolution of Chagas disease (American trypanosomiasis) control after 90 years since Carlos Chagas discovery. Mem Inst Oswaldo Cruz. 1999;94 Suppl 1:103–21. [DOI] [PubMed] [Google Scholar]
- 58.Kirchhoff LV. American trypanosomiasis (Chagas’ disease)--a tropical disease now in the United States. N Engl J Med. 1993;329:639–44. [DOI] [PubMed] [Google Scholar]
- 59.Williams-Blangero S, VandeBerg JL, Teixeira AR. Attitudes towards Chagas’ disease in an endemic Brazilian community. Cad Saude Publica. 1999;15:7–13. [DOI] [PubMed] [Google Scholar]
- 60.Goihman-Yahr M. American mucocutaneous leishmaniasis. Dermatol Clin. 1994;12:703–12. [PubMed] [Google Scholar]
- 61.Klaus S, Frankenburg S. Cutaneous leishmaniasis in the Middle East. Clin Dermatol. 1999;17:137–41; discussion 05–6. [DOI] [PubMed] [Google Scholar]
- 62.Medeiros ACR, Roselino AMF. Leishmaniose tegumentar americana: do histórico aos dias de hoje / Cutaneous american leishmaniasis: from the beginning until nowadays. An bras dermatol. 1999;74:329–36. [Google Scholar]
- 63.Molyneux DH, Morel C. Onchocerciasis and Chagas’ disease control: the evolution of control via applied research through changing development scenarios. Br Med Bull. 1998;54:327–39. [DOI] [PubMed] [Google Scholar]
- 64.Routh HB, Bhowmik KR. Filariasis. Dermatol Clin. 1994;12:719–27. [PubMed] [Google Scholar]
- 65.Paes-Leme C. Contribuicao ao estudo do Tokelau. Tese Facultade de Medicina do Rio de Janeiro Rio de Janeiro, Brazil, 1903. [Google Scholar]
- 66.Cunha PR. Sero Epidemiologia do Fogo Selvagem em um Foco Endemico Remanescente do Estado de Sao Paulo [Tese de Docencia]. Sao Paulo, Brazil.: Facultade de Medicina da Universidade de Sao Paulo.; 1987. [Google Scholar]
- 67.Da Silva LJ. A Agricultura Paulista a Partir de 1950. In Anexo 3: A Evolucion da Doenca de Chagas no Estado de Sao Paulo Funcraf-Hucite C Publisher., 1999. [Google Scholar]
- 68.Empinotti J, Diaz L, Martins C, Rivitti E, Sampaio S, Lombardi C, et al. Endemic pemphigus foliaceus in western Parana, Brazil (1976–1988). Cooperative Group for Fogo Selvagem Research. Br J Dermatol. 1990;123:431–7. [DOI] [PubMed] [Google Scholar]
- 69.Empinotti JC, Aoki V, Filgueira A, Sampaio SA, Rivitti EA, Sanches JA Jr., et al. Clinical and serological follow-up studies of endemic pemphigus foliaceus (fogo selvagem) in Western Parana, Brazil (2001–2002). Br J Dermatol. 2006;155:446–50. [DOI] [PubMed] [Google Scholar]
- 70.Proenca NG. Declinio do Penfigo Foliaceo no Estado de Sao Paulo. Rev Paulista Med (Brasil). 1977;89:97–100. [PubMed] [Google Scholar]
- 71.Silveira A, Vinhaes M. Elimination of vector-borne transmission of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94 Suppl 1:405–11. [DOI] [PubMed] [Google Scholar]
- 72.Vinhaes MC, Dias JC. [Chagas disease in Brazil]. Cad Saude Publica. 2000;16 Suppl 2:7–12. [PubMed] [Google Scholar]
- 73.Eaton DP, Diaz LA, Hans-Filho G, Santos VD, Aoki V, Friedman H, et al. Comparison of black fly species (Diptera: Simuliidae) on an Amerindian reservation with a high prevalence of fogo selvagem to neighboring disease-free sites in the State of Mato Grosso do Sul, Brazil. The Cooperative Group on Fogo Selvagem Research. J Med Entomol. 1998;35:120–31. [DOI] [PubMed] [Google Scholar]
- 74.Aoki V, Millikan RC, Rivitti EA, Hans-Filho G, Eaton DP, Warren SJ, et al. Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem). J Investig Dermatol Symp Proc. 2004;9:34–40. [DOI] [PubMed] [Google Scholar]
- 75.Hilario-Vargas J, Dasher DA, Li N, Aoki V, Hans-Filho G, dos Santos V, et al. Prevalence of anti-desmoglein-3 antibodies in endemic regions of Fogo selvagem in Brazil. J Invest Dermatol. 2006;126:2044–8. [DOI] [PubMed] [Google Scholar]
- 76.Ribeiro JM. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143–52. [PubMed] [Google Scholar]
- 77.Billingsley PF, Baird J, Mitchell JA, Drakeley C. Immune interactions between mosquitoes and their hosts. Parasite Immunol. 2006;28:143–53. [DOI] [PubMed] [Google Scholar]
- 78.Champagne DE, Wasserman HA, Kumar S, Singh S. Pharmacological and immunological properties of saliva of the blood-feeding insects Rhodnius prolixus and Aedes aegypti. Physiological Entomology. 2004;29:269–77. [Google Scholar]
- 79.Gillespie RD, Mbow ML, Titus RG. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol. 2000;22:319–31. [DOI] [PubMed] [Google Scholar]
- 80.Theodos CM, Ribeiro JM, Titus RG. Analysis of enhancing effect of sand fly saliva on Leishmania infection in mice. Infect Immun. 1991;59:1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manso EC, Morato-Castro FF, Yee CJ, Croce M, Palma MS, Croce J. Honeybee venom specific IgG subclass antibodies in Brazilian beekeepers and in patients allergic to bee stings. J Investig Allergol Clin Immunol. 1998;8:46–51. [PubMed] [Google Scholar]
- 82.Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurniawan A, Yazdanbakhsh M, van Ree R, Aalberse R, Selkirk ME, Partono F, et al. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993;150:3941–50. [PubMed] [Google Scholar]
- 84.Roselino A, Figueredo J, Kounga K, Reddy V, Lerner E. Serum IgG from Pemphigus Foliaceus Patients Reacts Against Maxadilan. J Invest Dermatol. 2001;117:460. [Google Scholar]
- 85.Arca B, Lombardo F, Valenzuela JG, Francischetti IM, Marinotti O, Coluzzi M, et al. An updated catalogue of salivary gland transcripts in the adult female mosquito, Anopheles gambiae. J Exp Biol. 2005;208:3971–86. [DOI] [PubMed] [Google Scholar]
- 86.Champagne DE, Nussenzveig RH, Ribeiro JM. Purification, partial characterization, and cloning of nitric oxide-carrying heme proteins (nitrophorins) from salivary glands of the blood-sucking insect Rhodnius prolixus. J Biol Chem. 1995;270:8691–5. [DOI] [PubMed] [Google Scholar]
- 87.Charlab R, Valenzuela JG, Rowton ED, Ribeiro JM. Toward an understanding of the biochemical and pharmacological complexity of the saliva of a hematophagous sand fly Lutzomyia longipalpis. Proc Natl Acad Sci U S A. 1999;96:15155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valenzuela JG, Belkaid Y, Rowton E, Ribeiro JM. The salivary apyrase of the blood-sucking sand fly Phlebotomus papatasi belongs to the novel Cimex family of apyrases. J Exp Biol. 2001;204:229–37. [DOI] [PubMed] [Google Scholar]
- 89.Valenzuela JG, Garfield M, Rowton ED, Pham VM. Identification of the most abundant secreted proteins from the salivary glands of the sand fly Lutzomyia longipalpis, vector of Leishmania chagasi. J Exp Biol. 2004;207:3717–29. [DOI] [PubMed] [Google Scholar]
- 90.Hemmer W, Focke M, Kolarich D, Dalik I, Gotz M, Jarisch R. Identification by immunoblot of venom glycoproteins displaying immunoglobulin E-binding N-glycans as cross-reactive allergens in honeybee and yellow jacket venom. Clin Exp Allergy. 2004;34:460–9. [DOI] [PubMed] [Google Scholar]
- 91.Volf P, Tesarova P, Nohynkova EN. Salivary proteins and glycoproteins in phlebotomine sandflies of various species, sex and age. Med Vet Entomol. 2000;14:251–6. [DOI] [PubMed] [Google Scholar]
- 92.Hill E, Broadbent ID, Chothia C, Pettitt J. Cadherin superfamily proteins in Caenorhabditis elegans and Drosophila melanogaster. J Mol Biol. 2001;305:1011–24. [DOI] [PubMed] [Google Scholar]
- 93.Barrett DJ, Ayoub EM. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986;63:127–34. [PMC free article] [PubMed] [Google Scholar]
- 94.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–70. [DOI] [PubMed] [Google Scholar]
- 95.Cobb BA, Kasper DL. Coming of age: carbohydrates and immunity. Eur J Immunol. 2005;35:352–6. [DOI] [PubMed] [Google Scholar]
- 96.Nedelec J, Boucraut J, Garnier JM, Bernard D, Rougon G. Evidence for autoimmune antibodies directed against embryonic neural cell adhesion molecules (N-CAM) in patients with group B meningitis. J Neuroimmunol. 1990;29:49–56. [DOI] [PubMed] [Google Scholar]


