Abstract
Chronic exposure to cadmium is known to be a risk factor for human prostate cancer. Despite over-whelming evidence of cadmium causing carcinogenicity in humans, the specific underlying molecular mechanisms that govern metal-induced cellular transformation remain unclear. Acute exposure (up to 72 hr) to cadmium induces apoptosis in normal prostate epithelial cells (RWPE-1), while chronic exposure (>1 year) transforms these cells to a malignant phenotype (cadmium-transformed prostate epithelial cells; CTPE). Increased expression of autophagy-regulated genes; Plac8, LC3B and Lamp-1; in CTPE cells was associated with cadmium-induced transformation. Increased expression of Plac8, a regulator of autophagosome/autolysosome fusion, facilitates the pro-survival function of autophagy and upregulation of pAKT(ser473) and NF-κβ, to allow CTPE to proliferate. Likewise, inhibition of Plac8 suppresses CTPE cell growth. Additionally, overexpression of Plac8 in RWPE-1 cells induces resistance to cadmium toxicity. Pharmacological inhibitors and an inducer of autophagy failed to affect Plac8 expression and CTPE cell viability, suggesting a unique role for Plac8 in cadmium-induced prostate epithelial cell transformation. These results support a role for Plac8 as an essential component in the cadmium-induced transformation of normal prostate epithelial cells to a cancerous state.
Keywords: Plac8, Cadmium, Autophagy, Prostate Cancer
1. Introduction
Exposure to cadmium is hazardous to human health [1] and chronic exposure is a risk for carcinogenesis [2]. Epidemiological data have correlated cadmium exposure with genitourinary, breast, lung and colon cancers, as well as hepatocellular carcinoma [3–6]. A number of signaling pathways are proposed to be components of mechanisms by which cadmium induces transformation, more specifically in prostate cancer [7, 8] .
Autophagy is a highly complex lysosomal-mediated degradation process that removes and recycles damaged organelles to maintain the quality of intracellular components. Autophagy-related genes (Atg) encode several serine-threonine kinases (including ULK) that comprise an integral part of the (Atg)/ULK complex, which is a key regulator of autophagy [9, 10]. This complex is required to initiate autophagosome formation, which is the first step in the autophagy process. Autophagosome formation eventually promotes autolysosomal degradation, via activation of the cytosolic microtubule-associated protein 1A/1B-light chain 3A (LC-3A) [11–13]. In mammalian cells, cytosolic LC-3B is lipidated and recruited to the membrane of the autophagosome where it plays an important role in the formation of the autophagosomes. LC-3B is thought to be a valid marker for autophagy in cancer [14]. Some Atg genes encode tumor suppressors that function in early stages of tumorigenesis. Some reports have concluded that the activation of autophagy may initiate either pro-survival machinery or induce pro-apoptotic signaling that decides the fate of the cell. Cadmium has been shown to induce oncogenic autophagy by suppressing cell death [15–17] and, on the other hand, induction of autophagy promotes cell death in neurons [18]. These studies suggest that autophagy plays dual regulatory roles in promoting cell survival in some cell types and cell death in others.
The cysteine-rich lysosomal protein Placental-specific 8 (Plac8) is targeted to the plasma membrane, a precursor of autophagosome-lysosome fusion. Plac8 function differs in by cell type; Plac8 overexpression induces apoptosis in human lymphocytes, and in fibroblasts it protects cells [19, 20]. In colon cancer cells, Plac8 induces epithelial-mesenchymal transition, and cell cycle regulation has been reported in pancreatic cancer cells [21, 22]. Uehara et al reported that increased Plac8 expression associated with proliferation and silencing Plac8 expression resulted in inhibition of prostate cancer growth in cell culture models [23]. Plac8 has also been shown to facilitate autophagosome-lysosome fusion by activating pro-survival function of autophagy in pancreatic cancer [24]. Plac8 facilitates autophagosome-autolysosome fusion without affecting the pH of lysosomes and degrades endocytic proteins, which is contrast to SNARE proteins (ex: SXT-7 and −18) [25].
To define the mechanism of cadmium-induced prostate carcinogenesis, normal prostate epithelial cells (RWPE-1) were continuously exposed to 10μM cadmium over a year. These cells, termed Cadmium induced Prostate Epithelial cells (CTPE), were resistant to cadmium toxicity, and formed cell mounds, indicative of cellular transformation. When CTPE cells were inoculated into Nude mice, poorly differentiated adenocarcinomas were observed in 90% of the mice within 4–6 weeks, which is consistent with tumors formed by human prostate epithelial cells [26–28]. Nearly 80% of these tumors invaded into the subdermal fat and connective tissue, which is an indication of aggressive behavior. Zymographic assays revealed that CTPE cells exhibit higher MMP-2 and MMP-9 secretions. Reduced expression of Bax and Bcl-2 was confirmed in CTPE cells, compared to RWPE-1 cells, suggesting that CTPE cells are resistant to apoptosis [29].
Chronic exposure to cadmium transforms normal prostate epithelial cells (RWPE-1) to a malignant state, however, the mechanism by which cadmium-induced transformation occurs in normal prostate epithelial cells remains unknown. In this study, we show that the induction of oncogenic autophagy in CTPE cells occurs due to the upregulation of Plac8. Plac8 overexpression in CTPE cells induces resistance to cadmium toxicity (i.e., cell growth inhibition) and cadmium-inhibited cell growth in CPTE cells with Plac8 knockdown. During chronic cadmium exposure to RWPE-1 cells, there was a gradual increase in Plac8 expression, suggesting that Plac8 is a driver of cadmium-induced prostate carcinogenesis.
2. Materials and Methods
2.1. Cell lines and reagents
Human normal prostate epithelial cells (RWPE-1) were purchased from the American Type Culture Collection (Manassas, VA, USA). CTPE cells were a gift from Dr. Mike Waalkes, NIEHS. RWPE-1 and CTPE cells were maintained in keratinocyte serum-free medium containing L-glutamine, EGF, BPE, supplemented with 10% fetal bovine serum, and 1% antibiotic and antimycotic solution in a humidified atmosphere of 5% CO2 at 37°C. Cadmium was purchased from Sigma Chemical Co (St. Louis, MO). BD Matrigel (high concentration) was purchased from BD Biosciences (San Jose, CA). Chloroquine (C6628), rapamycin (R8781), SBI-0206965 (SML1540) and Bafilomycin A1 (B1793) were purchased from Sigma Chemicals.
2.2. Cell viability, apoptosis and soft agar colony-formation assays
Cells were treated with cadmium (10 μM) or vehicle (referred as untreated (UT) in all figures) for up to 72 h. Cell viability was quantified using MTT assays (Cell Biolabs Inc., San Diego, CA, USA). Apoptosis assays were performed using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis kit-I (BD Pharmingen, San Diego, CA) as described previously [30]. Colony-formation assays were performed to monitor anchorage-independent growth using the CytoSelect™ 96-Well In Vitro Tumor Sensitivity Assay kit (Cell Biolabs Inc.). RWPE-1 and CTPE cells (5 × 103) were harvested and assayed, as previously described [31].
2.3. Whole transcriptome analysis
Total RNA was isolated from cadmium-treated and untreated RWPE-1 and CTPE cell lines in triplicate. Isolated RNA was checked for integrity (RIN>7) using the Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA) and quantified using a Qubit fluorometric assay (Thermo Fisher Scientific, Waltham, MA). Five hundred ng of total RNA was depleted of ribosomal RNA using the Illumina Ribo-Zero Gold rRNA Removal Kit (Human/Mouse/Rat) (Illumina, San Diego, CA). The depleted RNA was ligated with Illumina barcodes and adapters following the Illumina TruSeq Stranded Total RNA library preparation kit. All of the samples were pooled and sequenced using the NextSeq 500/550 High Output v2 75-cycle kit (Illumina) on the Illumina NextSeq platform. Upon sequencing completion, the resulting FastQ files were created on the Illumina BaseSpace server.
2.4. Protein extraction and Western blotting
RWPE-1 and CTPE cells were seeded in 6-well plates and incubated for 24 h and then treated with cadmium (10 μM) for up to 72h. Western blotting was performed using specific antibodies against: Atg3, Atg7, Atg12, LC3A, LC3B (Autophagy antibody sampler kit #4445, Cell Signaling, Danvers, MA), BAX, BCL-2, Plac8, Lamp-1, pAKTS473, p65, and cleaved PARP (Cell Signaling) STX-8, STX-17 (EMD Millipore, Norwood, OH)and β-actin (Santa Cruz Biotechnology, Dallas, TX). Protein-antibody complexes were visualized using enhanced chemiluminescence as previously described [31].
2.5. Real-time quantitative PCR
Total RNA was isolated from untreated- and cadmium-treated RWPE-1 and CTPE cells using Qiagen’s RNeasy Kit and 1 μg RNA was used for cDNA synthesis using the Applied Biosystems cDNA synthesis kit using SYBR Green supermix (Quiagen Inc., City, CA, USA), Quantitative RT-PCR was performed as previously described [30].
2.6. siRNA transfection
RWPE-1 and CTPE cells were seeded in 6-well plates at a density of 3 × 105 cells/well. After a 24 h incubation, cells were transiently transfected with siRNA specific for Plac8 or a control siRNA, as previously described [31].
2.7. Immunofluorescence analysis
Transfected RWPE-1 and CTPE cells were seeded on glass coverslips and allowed to attach and grow to 60% confluence as previously described [30]. Following treatment with vehicle or cadmium for 24 h, cells were washed and then incubated with Plac8 or LC3-B antibodies, followed by secondary antibodies conjugated to Alexa Fluor 488 (Green) to detect the localization and expression of the target proteins. The location of the antigen-antibody complexes were visualized using a Nikon laser scanning confocal microscope (Nikon Instruments Inc., Melville, NY).
2.8. Xenograft studies
Animals were housed under pathogen-free conditions, and experiments performed in accordance with the Institutional Animal Care & Use Committee (IACUC) and approved by the University of Louisville. Balb/c athymic nude mice (nu/nu) were purchased from The Jackson Laboratory and used at 6 weeks of age. For subcutaneous tumor xenograft studies, RWPE-1 and CTPE cells (1.5 × 105) in 50 μL (final volume) of Matrigel (1:1) in phosphate-buffered saline were injected into separate flanks of mice (n = 10). Mice were monitored twice weekly, and the tumor volumes were measured once per week.
2.9. Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0a software (GraphPad Software, Inc., La Jolla, CA). Results are expressed as mean ± SEM. Data sets were compared using a two-tailed unpaired Student’s t-test. Statistical significance was set at p <0.05.
3. Results
3.1. Effect of acute and chronic exposure of cadmium in prostate epithelial cells
First, we explored the acute toxicity of cadmium (10 μM) for up to 72 h in exposed RWPE-1 and CTPE cells. Significant growth inhibition was observed in RWPE-1 cells in a time-dependent manner (p<0.05–0.001), compared with vehicle-treated cells. In contrast, CTPE cell growth was not significantly affected, which suggested that CTPE cells were resistant to cadmium toxicity (Fig. 1A).
Figure 1. Cadmium cytotoxicity in RWPE-1 and CTPE.
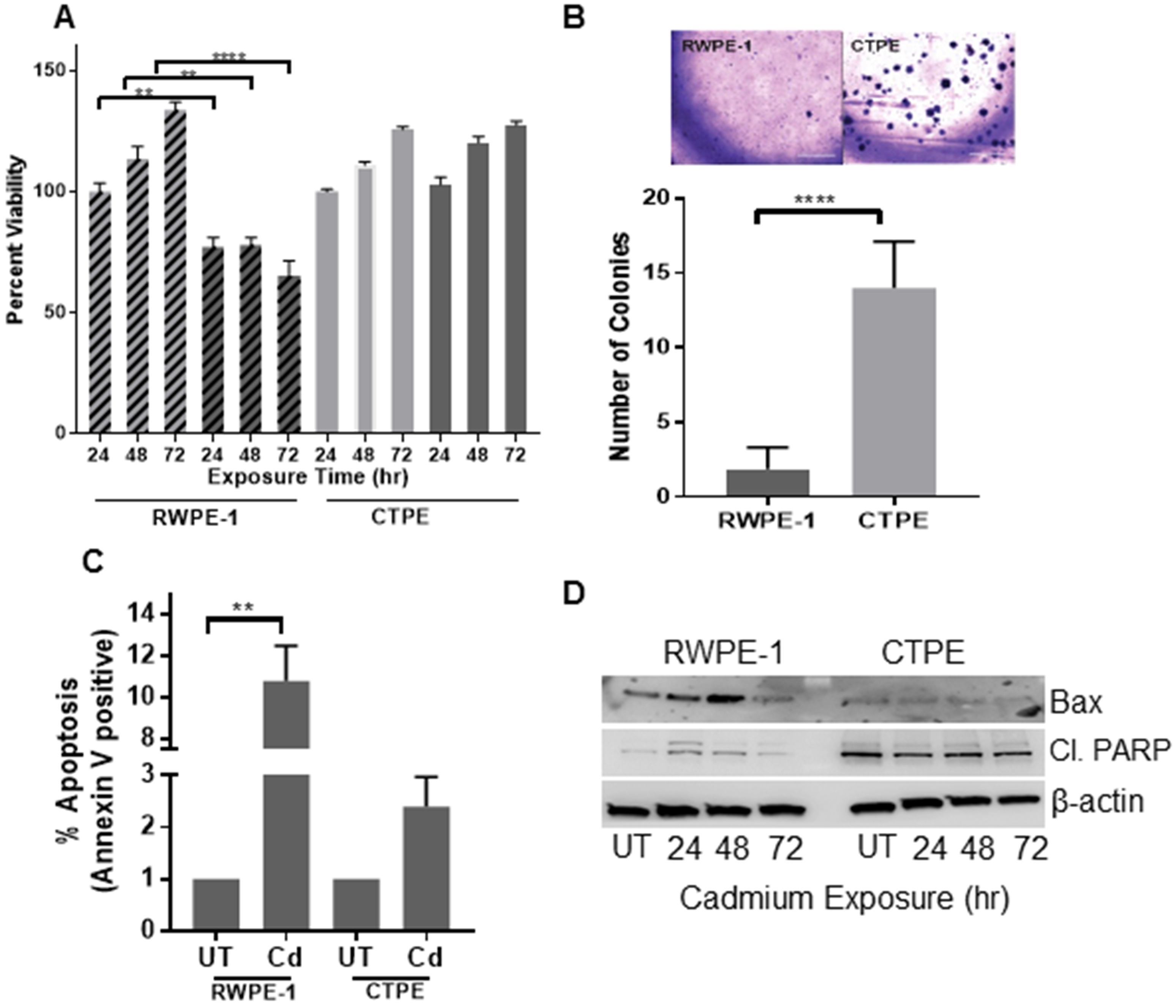
(A) Cell viability of RWPE-1 (stripe bars) and CTPE (solid bars) cells treated with vehicle (light color) cadmium for 24, 48 and 72 h (dark color) was determined. (B) To assess anchorage-independent growth, 1 × 103 RWPE-1 or CTPE cells were grown in soft agar for 10–15 days. Colonies were stained with crystal violet and manually counted. (C) The effect of cadmium exposure on apoptosis was determined using an Annexin V assay. Apoptosis levels in RWPE-1 and CTPE cells were measured by flow cytometry 24 h after cadmium treatment. (D) The detection of apoptotic proteins; Cleaved PARP (Cl. PARP) and BAX; in cadmium-treated RWPE-1 and CTPE cells were measured by Western blotting; β-actin was used as a loading control. Data are expressed as mean ± SEM of two independent experiments conducted in triplicate. The Student’s t-test was used to calculate statistical significance between the vehicle control and treatment at each time point. **p < 0.01, ***p < 0.001, ****p < 0.0001
To determine if growth inhibition by cadmium in RWPE-1 cells was due to the induction of apoptosis, Annexin V-FITC apoptotic assays were performed. A significant increase in cell death (12%) was observed in cadmium-treated RWPE-1 cells, compared with CTPE cells (2%) (Fig. 1C). The apoptotic markers Bax and cleaved-PARP were also measured. No significant changes of either BAX or cleaved-PARP were observed in cadmium-treated CTPE cells. In contrast, higher levels of expression of both pro-apoptotic proteins were observed in cadmium-treated RWPE-1 cells (Fig. 1D). Combined, these results confirm the sensitivity and resistance of RWPE-1 and CTPE cells, respectively, to acute cadmium exposure.
To confirm CTPE cell transformation, we used the soft agar colony-formation assay, which is a stringent test for malignant cell transformation. CTPE cells exhibited a significant (p<0.001) increase in colony formation, compared with RWPE-1 cells, which produced few smaller-sized colonies (Fig. 1B). Finally, we subcutaneously injected 106 CTPE and 107 RWPE-1 cells into separate flanks of athymic nude mice to further assess the transformation status of CTPE cells. CTPE tumors exhibited rapid growth, while no tumor growth was observed with RWPE-1 cells (Fig. 2).
Figure 2. Formation of tumors in xenografts in nude mice.
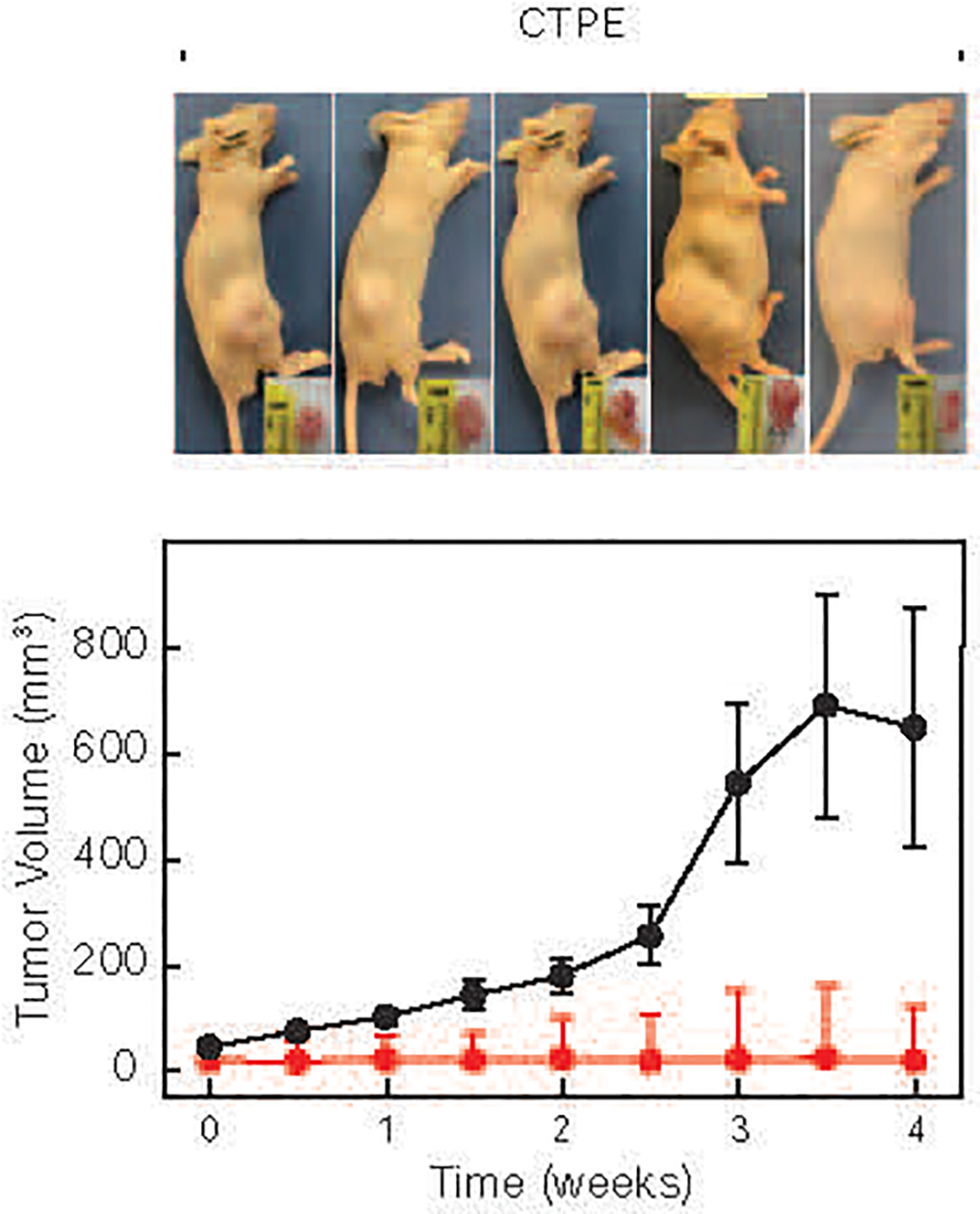
RWPE-1 (red line) and CTPE (black line) cells were injected into nude mice and tumor growth monitored twice a week. Tumor and mouse images were obtained 31 days after injection.
3.2. Identification of molecular targets of cadmium in non-transformed and transformed prostate epithelial cells
To gain mechanistic insights into cadmium-induced transformation, RNAseq analyses were performed on RWPE-1 and CTPE cells. Differences between RWPE-1 and CTPE cells in autophagy-regulated genes (Plac8, LC3B, STX8, STX17 and Lamp-1) were observed, suggesting that autophagy plays a major role in cadmium-induced transformation (Fig. 3A). Most notably, Plac8 was highly expressed in CTPE cells (≥100 fold), with low levels in RWPE-1 cells. We confirmed the changes protein expression of autophagy markers, pro-survival proteins and transcriptional factors in CTPE cells. Lysosomal proteins, Lamp-1, STX8, STX17, Plac8 and LC3B were highly expressed in CTPE cells, compared with RWPE-1 cells (Fig. 3B). Additionally, higher levels of pAKTS473 and p65 protein, and NFκB activity were observed in CTPE cells compared to RWPE-1 cells (Figs. 3B and 3C). To determine levels of autophagosomes-lysosomes fusion, we performed localized immunofluorescence assays. Plac8 co-localized with LC3B in CTPE cells, which suggested fusion. However, in RWPE-1 cells, low levels of both proteins were expressed (Fig. 3D).
Figure 3. Molecular characterization of RWPE-1 and CTPE cells.
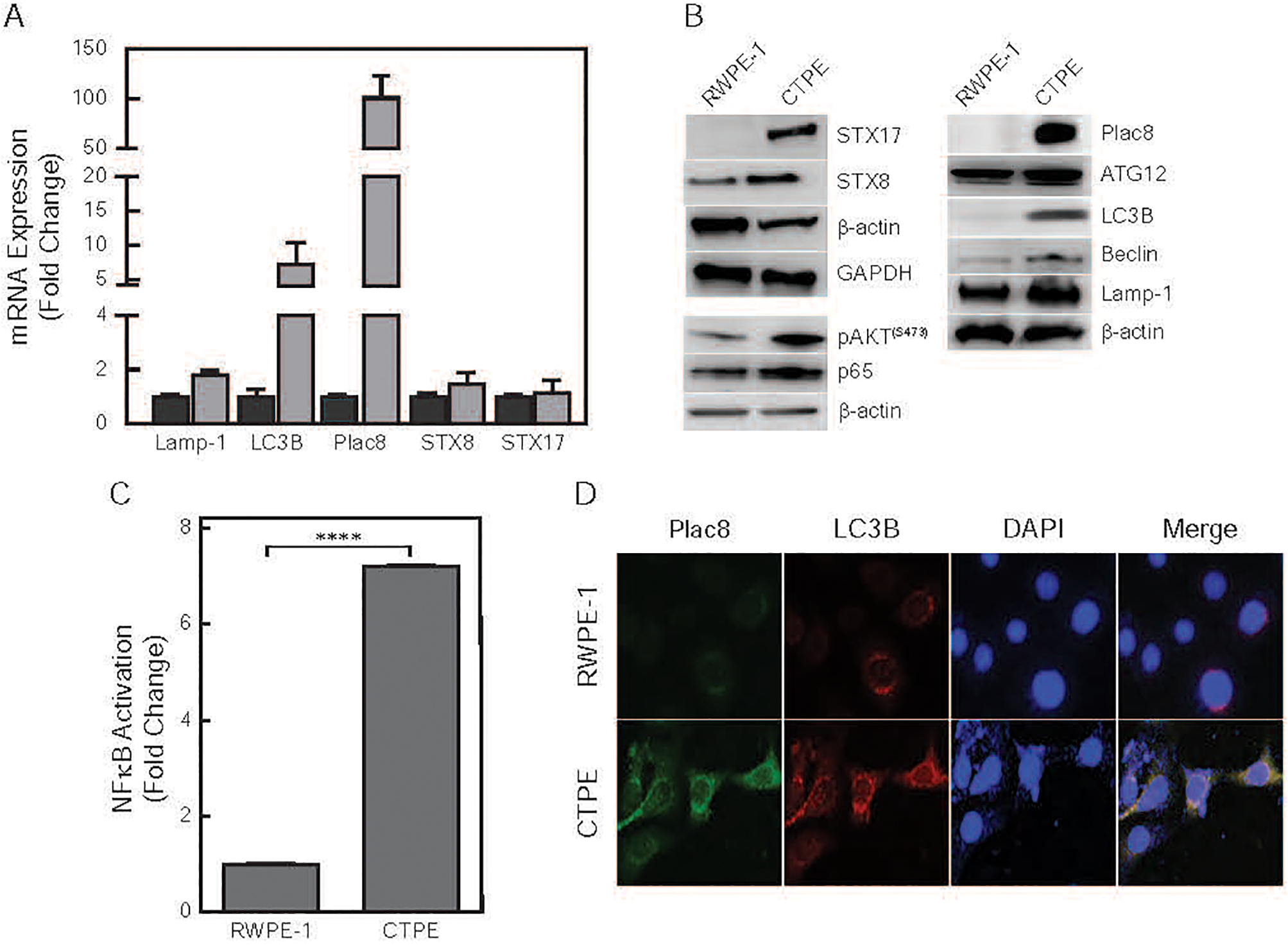
(A) Total RNA was isolated from untreated RWPE-1 (dark gray) and CTPE (light gray) cells. Differences in steady-state mRNA levels were determined following RNAseq and are presented as fold change relative to RWPE-1 cells. (B) Western blot analysis of NFκB and autophagy proteins in whole cell lysates. β-actin and GAPDH were used as loading controls. (C) Nuclear factions of RWPE-1 and CTPE were extracted and subjected to NFκB p65 Transcription Factor Assay by EMSA analysis. Student’s t-test was used to calculate the statistical significance between cell lines ****p < 0.0001 (D) Immunofluorescence was used to measure the intracellular location of Plac8 and LAMP-1 in RWPE-1 and CTPE cells.
3.3. The role of Plac8 in cadmium-induced transformation,
To further define the role of Plac8 in cadmium-induced transformation, CTPE cells were transiently transfected with either scrambled/control siRNA or Plac8-siRNA, and then treated with cadmium for 24h. Knocking down Plac8 expression significantly (p<0.01) inhibited CTPE cell growth. In the presence of cadmium, it further inhibited growth (p=0.001), which was not observed in control transfected cells (Fig. 4A). Western blot analysis confirmed lower levels of Plac8 in Plac8-siRNA transfected cells, which was not altered in control transfected cells in either the presence or absence of cadmium (Fig. 4A).
Figure 4. Functional role of Plac8.
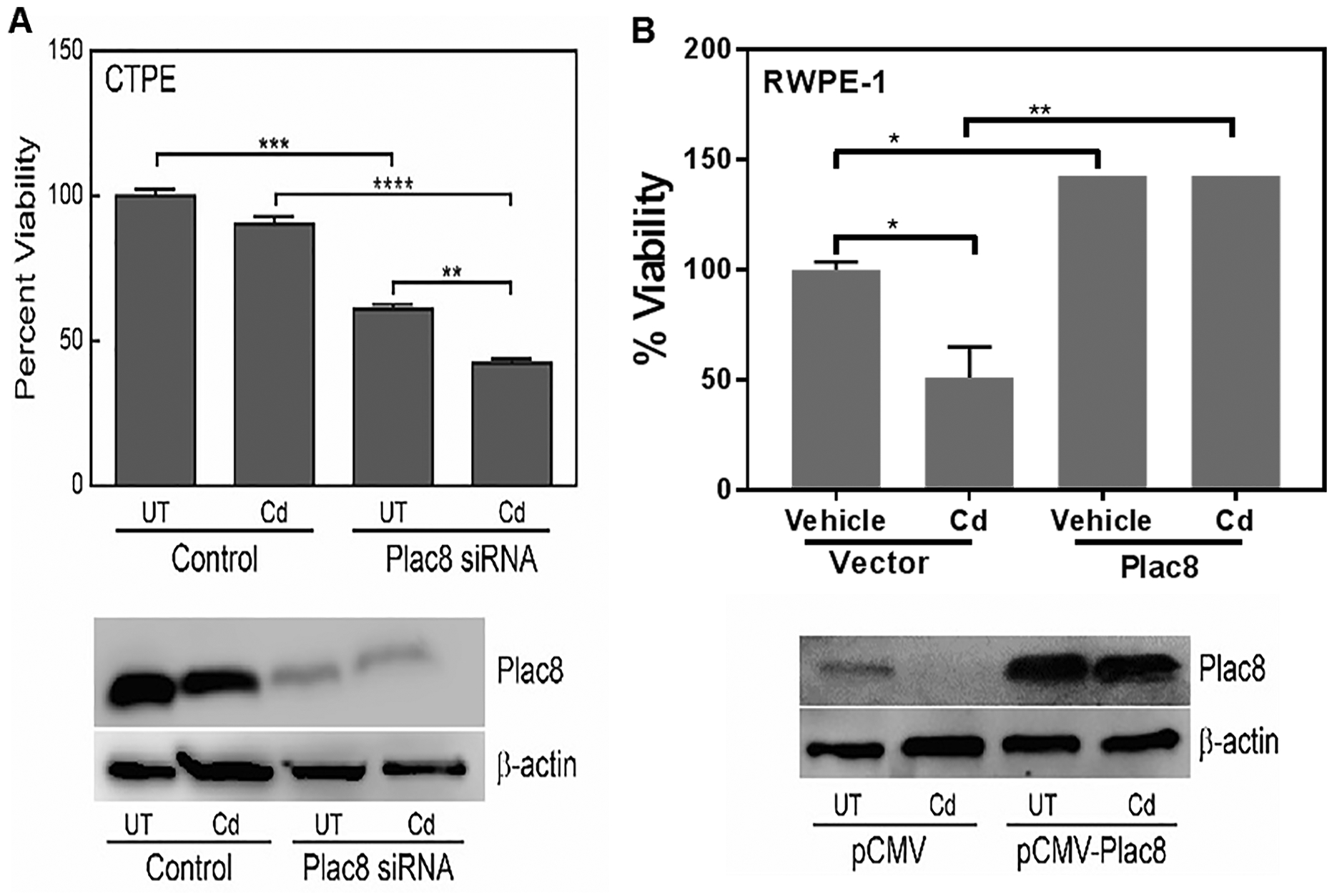
(A) CTPE cells where Plac8 expression was reduced using siRNA (Plac8siRNA) or a non-specific/scrambled siRNA (Control) were treated with cadmium (Cd) or vehicle (UT) for 24 h; cell viability was then determined. (B) RWPE-1 cells where Plac8 was overexpressed (pCMV-Plac8) were treated with cadmium for 24 h and cell viability was determined. Western blots present changes in Plac8 expression due to RNAi or overexpression. Student’s t-tests were used to calculate statistically significance differences between the various treatment groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Overexpression of Plac8 in RWPE-1 cells significantly increased resistance to cadmium toxicity; to a level similar to CTPE cells (Fig. 4B). Western blot analysis confirmed the overexpression of Plac8 in RWPE-1 cells, did not alter Plac8 expression in transfected cells (pCMV-Plac8) in either the presence or absence of cadmium (Fig. 4B). These results confirm an essential role for Plac8 in resistance to cadmium toxicity.
In cadmium-induced transformation in RWPE-1 cells (2 and 4 months), a time-dependent increase in colony formation with cadmium treatment was observed that positively correlated with Plac8 protein (p=0.0001) (Figs. 5A and 5B) and mRNA levels (Fig. 5C). Immunofluorescence analysis confirmed a time-dependent accumulation of Plac8 in lysosomal compartments of transforming cells (Fig. 5D), which corresponded to proliferation of transforming cells (data not shown). These results clearly support a model where Plac8 is a driving force in the cadmium-induced transformation of prostate epithelial cells.
Figure 5. Plac8 expression during cadmium-induced RWPE-1 transformation.
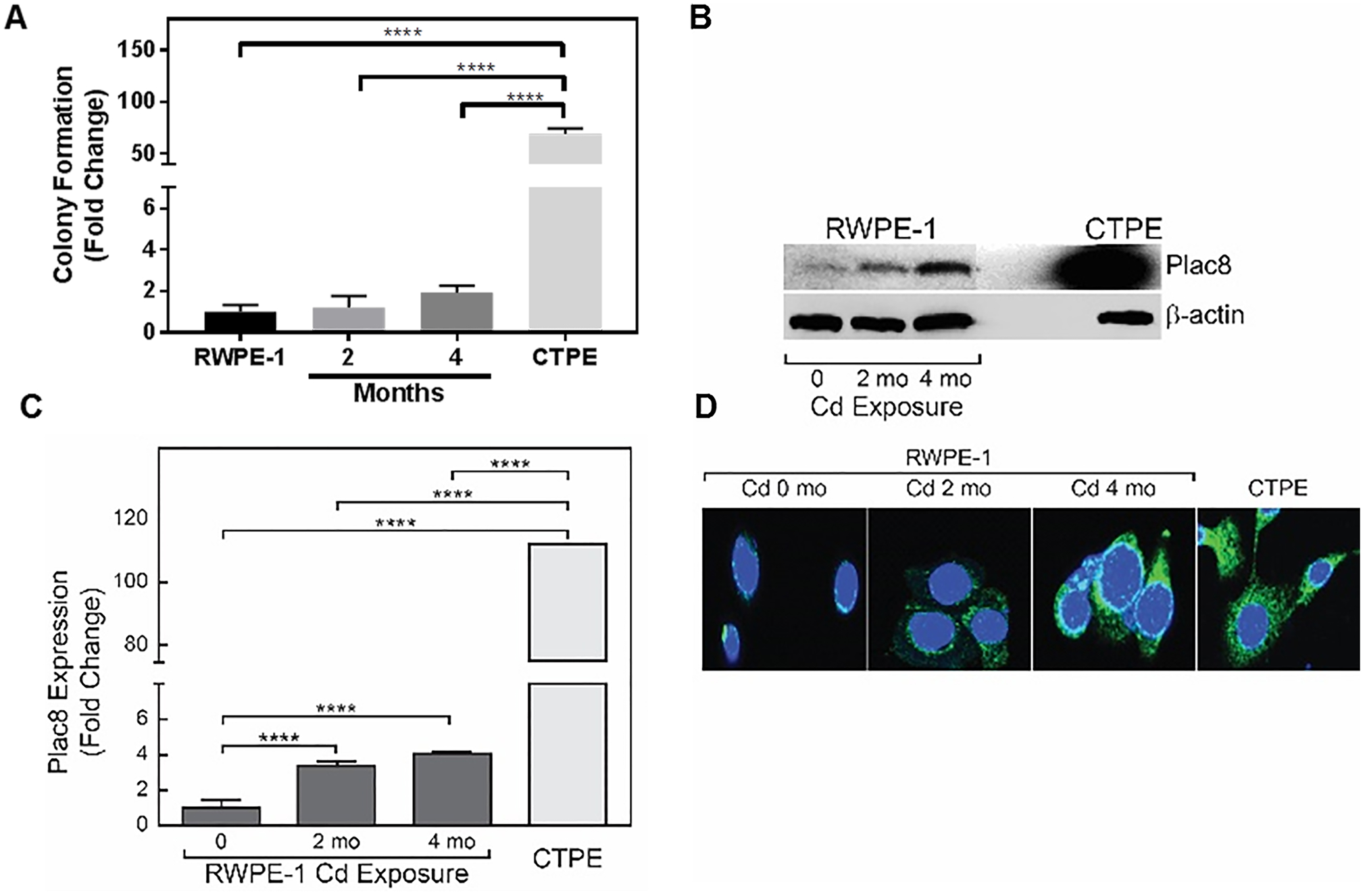
(A) RWPE-1 cells treated with cadmium for 0 (untreated), 2 and 4 months and non-treated CTPE cells were grown in soft agar for 10–15 days. Colonies were stained with crystal violet and counted. (B) Levels of Plac8 expression in RWPE-1 cells as a function of cadmium exposure time (months, mo) and non-treated CTPE cells were measured in whole cell lysates by Western blot analysis; β-actin was used as a loading control. (C) Total RNA was extracted and qRT-PCR was performed to examine steady state levels of Plac8 mRNA expression in RWPE-1 exposed to cadmium for 0 (untreated) 2 and 4 months and CTPE cells. (D) Intracellular location of Plac8 was determined by immunohistochemistry in cadmium-treated and non-treated RWPE-1 and CTPE cells. Student’s t-tests were used to calculate statistically significance differences between the various treatment groups. *p < 0.05, **p < 0.01, ****p < 0.0001
Pharmacological inhibitors of autophagy were used to determine the pro-survival role of autophagy in the phenotypic changes in CTPE cells. Bafilomycin A1 and chloroquine are known inhibitors of autophagy that act by preventing the fusion of autophagosomes with lysosomes [32–34]. Rapamycin an inhibitor of mTOR signaling in turn blocks cell growth and proliferation, similar to the ULK1 inhibitor, SBI-0206965 [35, 36].
CTPE cells were treated for 24 h with the inhibitors and inducers at their respective IC50s (BAF; 200nM, CQ; 50μM, RAP; 500 nM, SBI; 200nM). CTPE cell viability was not significantly affected by the pharmacological inhibitors or inducers (Fig. 6A), which correlated with lack of change in Plac8 expression (Fig. 6B). However, viability of CTPE cells significantly reduces when treated with Plac8-siRNA (Fig. 4A). These results suggest that Plac8 is a unique component in cadmium-induced transformation in prostate epithelial cells and that the inhibition of Plac8 is necessary for growth inhibition in CTPE cells.
Figure 6. Effects of autophagy inhibitors on CTPE cell growth.
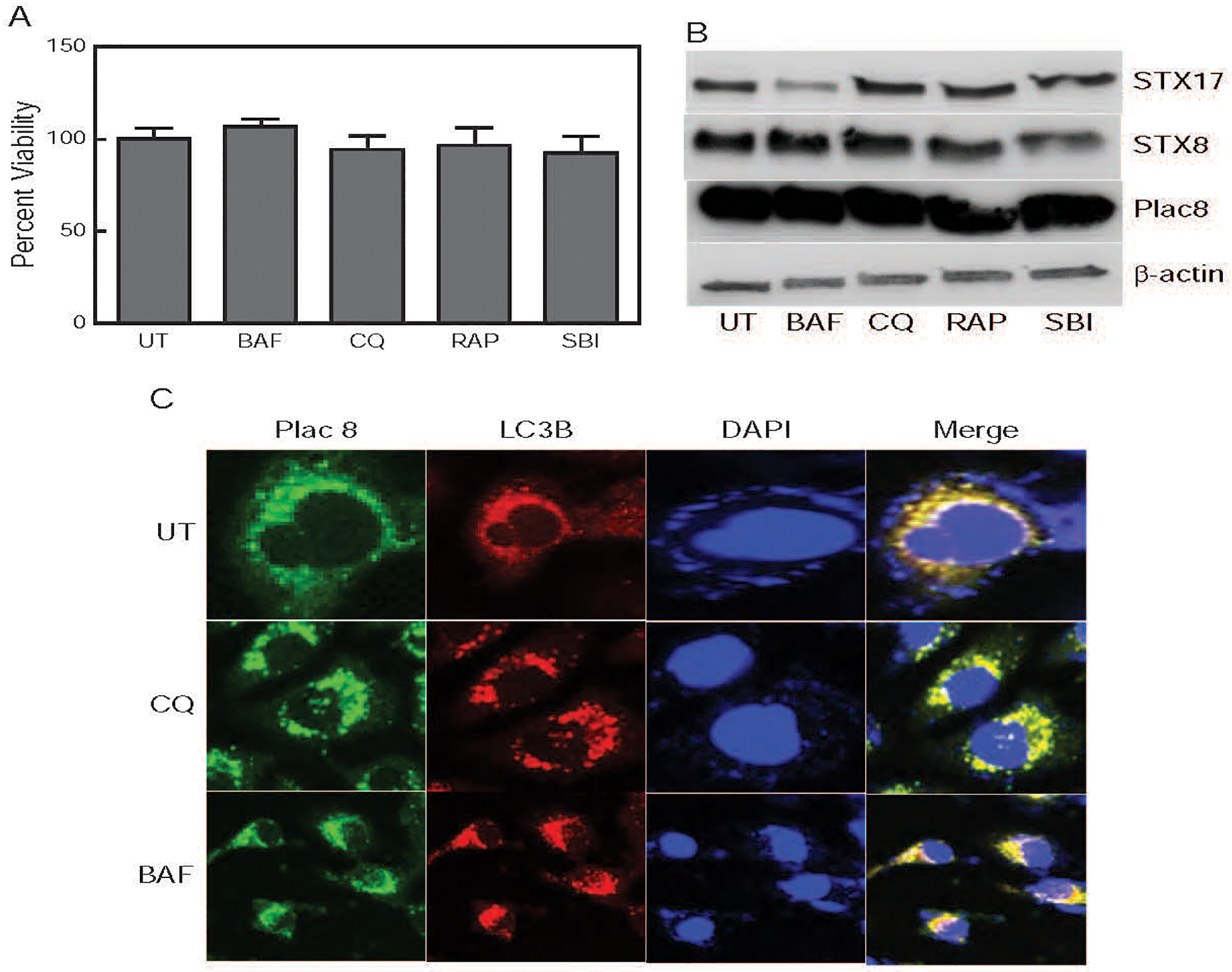
(A) CTPE cells were treated with pharmacological autophagy inhibitors; Bafilomycin A1 (BAF) and Chloroquine (CQ), and/or autophagy inducers; rapamycin (RAP) and SBI-0206965 (SBI); or vehicle (UT) for 24 h and cell viability was determined. (B) Levels of STX17, STX8 and Plac8 expression following inhibitor treatment in whole cell lysates were determine by Western blot analysis; β-actin was used as a loading control. (C) CTPE cells were treated with vehicle, BAF or CQ for 24h and immunofluorescence analysis of Plac8 and LC3B performed. Merge figures show autophagosome and autolysosome fusion.
Next, we determined whether inhibition of Plac8 would block autophagosome and autolysosome fusion in CTPE cells. Immunofluorescence analysis was performed using Plac8 (lysosome marker) and LC3B (phagosome marker) in CTPE cells treated with vehicle, Baf or CQ for 24h. Chloroquine and Baf failed to block phagosome and lysosome fusion, which resulted in induction of autophagy (Fig. 6C). The above results (Fig. 6A&C) strongly suggest that Plac8 inhibition is necessary to suppress the growth of CTPE cells.
3.4. The pro-survival mechanism of CTPE cells
To further define the role of pro-survival function of autophagy, changes in the expression of the pro-survival proteins; pAKTS473, p65, Bcl-2; and autophagy target proteins; Plac8, Lamp-1, STX8, STX17 and LC3B; in cadmium-treated and untreated CTPE and RWPE-1 cells were determined. Significant increases in the levels of pAKTS473, p65, Plac8, STX8, STX17 and LC3B proteins and mRNAs were observed in cadmium-treated CTPE cells, but not RWPE-1 cells (Figs. 7A and 7C). No significant changes were seen in the expression of Atg-3, −7, −12, p62 and Nrf2 (data not shown). Vehicle-treated and cadmium-treated RWPE-1 cells exhibited low levels of Plac8 and LC3B. In contrast, higher basal expression of Plac8, as well as cadmium-treated CTPE cells were observed (Fig. 7B). In RWPE-1 cells, little or no fusion of phagosomes (Plac8) and lysosomes (LC3B) was observed in control or cadmium treated cells. In contrast, higher basal levels of autophagosome/lysosome fusion in CTPE cells that increased with cadmium treatment were observed (Fig. 7B). This suggests that the induction of pro-survival function of autophagy protects CTPE cells from cadmium toxicity to allow them to proliferate. These results support our overall model in which pro-survival function of autophagy and Plac8 have important functions in cadmium-mediated prostate epithelial cell transformation and/or proliferation.
Figure 7. Effect of cadmium exposure on apoptosis and autophagy in RWPE-1 and CTPE cells.
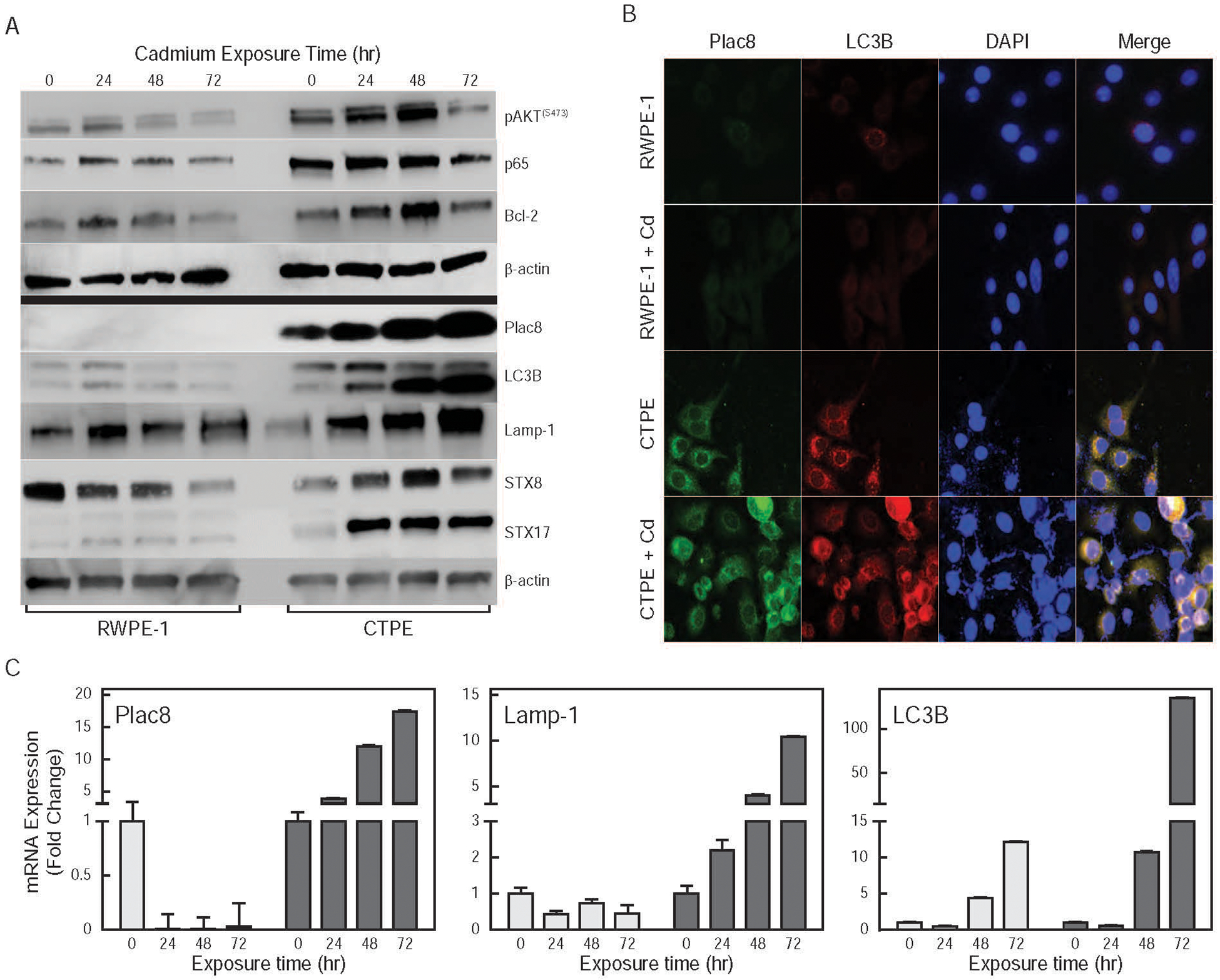
(A) Effects of exposure to cadmium for 0, 24, 48 and 72 hr on apoptosis; pAKTS473, p65 and Bcl-2; and autophagy; Plac8, LC3B, Lamp-1, STX8 and STX17; markers were determined in whole cell lysates by Western blot analysis; β-actin was used as a loading control. (B) Effect of cadmium exposure on levels and intracellular localization of Plac8 and LC3B in RWPE-1 and CTPE cells. (C) Effects of exposure to cadmium for 0, 24, 48 and 72 hr on steady state Plac8, Lamp-1 and LC3B mRNA levels in RWPE-1 (light bars) and CTPE (dark bars) were measured by qRT-PCR.
4. Discussion
It is clear that cadmium causes carcinogenicity in humans and that exposure to cadmium can induce autophagy in many cell types [37, 38]. The specific molecular mechanisms that govern metal induced cellular transformation, however remains unclear. In this study, cadmium exposure causes the induction of Plac8, and activation of AKT signaling which initiates oncogenic autophagy making the cells more aggressive and malignant.
In the current study, we find that the induction of pro-survival function of autophagy protects cells from environmental insults to facilitate the proliferation of CTPE cells. Gene expression analysis confirmed that autophagy-regulated genes were highly expressed in transformed cells; with Plac8 showing the greatest increase in both mRNA and protein expression. Increases in the levels of mRNA and protein of another marker of autophagy, LC3B, was also observed in transformed cells. This further supports the initiation of phagosome as an early event of autophagy. The lysosomal protein Plac8 is currently being examined as an effector in cellular transformation. The function of Plac8 differs based on the cell type; ectopic expression Plac8 protects fibroblasts but in epithelial cells it induces apoptosis [19, 20]. In colon cancer, Plac8 induces epithelial-mesenchymal transition, and cell cycle regulation is reported in pancreatic cancer cells [21, 22]. It has been shown that Plac8 plays a role in autophagosome-lysosome fusion by initiating oncogenic-autophagy in pancreatic cancer [24]. In prostate cancer, induction of Plac8 correlated with increased proliferation and silencing Plac8 resulted in growth inhibition in cell culture models [23]. We have shown that silencing Plac8 expression results in the loss of the protective function of autophagy and induction of cell death in CTPE cells, on the other hand, Plac8 overexpressing RWPE-1 cells are resistant to cadmium treatment. This was further confirmed with Annexin V binging assay, which confirms that cadmium exposed RWPE-1 cells show large induction of apoptosis.
Plac8 plays a major role in cadmium-induced transformation of RWPE-1 cells. Plac8 localizes to the inner surface of the plasma membrane and after activation it interacts with other cellular components to execute its functions: autophagy, cell cycle arrest and apoptosis [21]. In our studies, Plac8 expression is correlated with cell proliferation and the transformation of normal prostate epithelial cells to the malignant state during chronic exposure to cadmium. Silencing Plac8 expression results in growth inhibition in CTPE cells, while overexpression of Plac8 causes resistance to cadmium toxicity in non-transformed prostate epithelial cells. These results indicate an essential role of Plac8 in cellular transformation. Plac8 expression correlated with autophagosome and lysosome fusion, and the induction of pro-survival function of autophagy in cadmium-transformed cells. Furthermore, immunofluorescence analysis showed Plac8 and the phagosome marker LC3B in close proximity in transformed cells. We also observed a higher expression (mRNA and protein) of autophagy markers Atg-family and SNARE proteins (STX8, STX17) in cadmium-induced transformed cells. These results indicate that the autophagy machinery is active and may protect cells from cadmium toxicity allowing the proliferation of transformed prostate epithelial cells.
We performed proliferation and colony-formation analysis and xenograft tumor growth studies to confirm transformation in CTPE cells. In transformed cells, there were higher levels of expression of the pro-survival markers pAKTS473, p65 and Bcl-2, compared with non-transformed cells. This observation is similar to results to that reported when RWPE-1 and CTPE cells were examined [39]. Cadmium activates PI3K/AKT signaling in different cell culture models, including prostate cancer [40–42]. A number of studies have shown that the induction of reactive oxygen species (ROS) activates PI3K/AKT signaling to induce cell proliferation and tumor growth in cadmium-transformed cells [39, 41]. The overexpression of antioxidants; catalase, superoxide dismutase-1 (SOD1) or SOD2; diminishes cadmium-induced AKT activation and results in growth inhibition [41]. Similarly, the induction of ROS is correlated with NFκB activation, which inhibits apoptosis in cadmium-treated cells. The inhibition of NFκB -facilitates cadmium-induced apoptosis, suggests that NFκB activation plays a major role in cadmium-induced cell proliferation [43, 44]. Waalkes and others have reported that Bcl-2 expression was five times higher in CTPE cells compared to non-transformed RWPE-1 cells and that its expression conferred resistance to cell death [29, 39]. Induction of Bcl-2 overrides the pro-apoptotic function of JNK-mediated cell death by phosphorylation of JNK in CTPE cells [45].
Cross-talk between apoptosis and autophagy cooperate leading to cell death. An outcome of this is if one program is blocked, the other takes over. In a scenario where autophagy leads to cell death, the death programs serve as back-up to ensure efficient cell death. This cross-talk may be mistaken for a contributing role in apoptotic cell death, wherein autophagy may only be necessary for a particular hallmark of apoptosis. Hence, cell death assays such as Annexin V binding, which examines an ATP-dependent feature should be supplemented with other independent criteria [46]. The results presented in this report support a model where the upregulation of Plac8 is responsible for the induction of pro-survival function of autophagy, which facilities survival and proliferation of CTPE cells. Acute exposure to cadmium fails to induce Plac8 expression and as a result, cadmium inhibits growth in normal prostate epithelial cells. Chronic cadmium exposure however causes Plac8 induction and subsequent downstream effects on autophagy; fusion of autophagosome and autolysosome. This leads to protection against cadmium-induced toxicity and cell proliferation.
Autophagy acts as an adaptive mechanism to protect cells from environmental stress, serum starvation and oxidative stress to promote cell survival. We show that the induction of autophagy correlates with the proliferation of CTPE cells. In contrast, other studies report a correlation between the induction of autophagy and cell death [47, 48]. We used four different autophagy inhibitors on CTPE cells to delineate the role of autophagy in cell proliferation and if they affected Plac8 expression in transformed cells. None of the inhibitor affected cell growth or Plac8 expression. These results contradict previous reports where chloroquine and Bafilomycin A1 prevent autophagosome/autolysosome fusion [32–34]. This difference may be due to several lysosome genes regulating autophagosome/lysosome fusion and the expression of these genes being absent in CTPE cells. Additionally, Plac8 may be the major regulator of autophagosome/lysosome fusion in these cells, which is not affected by these inhibitors (Fig. 6). To further explore this scenario, we analyzed R-SNAREs, a sub group of SNARE proteins localized in the donor membrane that are involved in the regulation of autophagosome-lysosome fusion [49]. We did not detect R-SNARE proteins (VAMP/synaptobrevin), suggesting that these proteins may not play any role in transformed cells.
In summary, the oncogenic role of Plac8 in inducing the pro-survival function of autophagy protects the cells from environmental stress and aids in the transformation in prostate epithelial cells during chronic exposure of cadmium. The absence of Plac8 in normal prostate epithelial cells and its gradual increase in expression during transformation suggests that Plac8 may be a driving force in cadmium-induced transformation. Additionally, it suggests that specifically targeting Plac8 may affect prostate carcinogenesis in humans, and that Plac8 activation may be used as a biomarker for the early detection of prostate cancer in cadmium-exposed populations.
Acknowledgements:
This work was supported by collaborative matching grant and JHFEREG from college of medicine, University of Louisville to CD and JF. The authors thank Trinath Das for RNA extraction to perform RNA seq analysis.
Footnotes
Conflict of Interest
The authors declare that no conflict of interests in relation to the work described in the study. In addition it is an original research and this work is not published or under consideration anywhere.
7. References
- 1.Meeting of the IARC working group on beryllium, cadmium, mercury and exposures in the glass manufacturing industry, Scand J Work Environ Health, 19 (1993) 360–363. [DOI] [PubMed] [Google Scholar]
- 2.Roy SS, Mahapatra R, Rath S, Bajpai A, Singh V, Rath S, Nair N, Tripathy P, Gope RK, Sinha R, Costello A, Pagel C, Prost A, Improved neonatal survival after participatory learning and action with women’s groups: a prospective study in rural eastern India, Bull World Health Organ, 91 (2013) 426–433B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjellstrom T, Friberg L, Rahnster B, Mortality and cancer morbidity among cadmium-exposed workers, Environ Health Perspect, 28 (1979) 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takenaka S, Oldiges H, Konig H, Hochrainer D, Oberdorster G, Carcinogenicity of cadmium chloride aerosols in W rats, J Natl Cancer Inst, 70 (1983) 367–373. [PubMed] [Google Scholar]
- 5.Waalkes MP, Rehm S, Perantoni AO, Coogan TP, Cadmium exposure in rats and tumours of the prostate, IARC Sci Publ, (1992) 391–400. [PubMed] [Google Scholar]
- 6.Julin B, Wolk A, Bergkvist L, Bottai M, Akesson A, Dietary cadmium exposure and risk of postmenopausal breast cancer: a population-based prospective cohort study, Cancer Res, 72 (2012) 1459–1466. [DOI] [PubMed] [Google Scholar]
- 7.Golovine K, Makhov P, Uzzo RG, Kutikov A, Kaplan DJ, Fox E, Kolenko VM, Cadmium down-regulates expression of XIAP at the post-transcriptional level in prostate cancer cells through an NF-kappaB-independent, proteasome-mediated mechanism, Mol Cancer, 9 (2010) 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye J, Wang S, Barger M, Castranova V, Shi X, Activation of androgen response element by cadmium: a potential mechanism for a carcinogenic effect of cadmium in the prostate, J Environ Pathol Toxicol Oncol, 19 (2000) 275–280. [PubMed] [Google Scholar]
- 9.Mizushima N, Yoshimori T, Ohsumi Y, The role of Atg proteins in autophagosome formation, Annu Rev Cell Dev Biol, 27 (2011) 107–132. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM, Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury, Autophagy, 10 (2014) 2208–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N, FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells, J. Cell Biol, 181 (2008) 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N, Atg101, a novel mammalian autophagy protein interacting with Atg13, Autophagy, 5 (2009) 973–979. [DOI] [PubMed] [Google Scholar]
- 13.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH, ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery, Mol. Biol. Cell, 20 (2009) 1992–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klionsky DJ, Emr SD, Autophagy as a regulated pathway of cellular degradation, Science, 290 (2000) 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chargui A, Zekri S, Jacquillet G, Rubera I, Ilie M, Belaid A, Duranton C, Tauc M, Hofman P, Poujeol P, El May MV, Mograbi B, Cadmium-induced autophagy in rat kidney: an early biomarker of subtoxic exposure, Toxicol. Sci, 121 (2011) 31–42. [DOI] [PubMed] [Google Scholar]
- 16.Chen CL, Lin CF, Chang WT, Huang WC, Teng CF, Lin YS, Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway, Blood, 111 (2008) 4365–4374. [DOI] [PubMed] [Google Scholar]
- 17.So KY, Ahn SG, Oh SH, Autophagy regulated by prolyl isomerase Pin1 and phospho-SerGSK3alphabeta involved in protection of oral squamous cell carcinoma against cadmium toxicity, Biochem Biophys Res Commun, 466 (2015) 541–546. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Liu L, Luo Y, Huang S, MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis, J. Neurochem, 105 (2008) 251–261. [DOI] [PubMed] [Google Scholar]
- 19.Mourtada-Maarabouni M, Watson D, Munir M, Farzaneh F, Williams GT, Apoptosis suppression by candidate oncogene PLAC8 is reversed in other cell types, Curr Cancer Drug Targets, 13 (2013) 80–91. [PubMed] [Google Scholar]
- 20.Rogulski K, Li Y, Rothermund K, Pu L, Watkins S, Yi F, Prochownik EV, Onzin, a c-Myc-repressed target, promotes survival and transformation by modulating the Akt-Mdm2-p53 pathway, Oncogene, 24 (2005) 7524–7541. [DOI] [PubMed] [Google Scholar]
- 21.Kaistha BP, Lorenz H, Schmidt H, Sipos B, Pawlak M, Gierke B, Kreider R, Lankat-Buttgereit B, Sauer M, Fiedler L, Krattenmacher A, Geisel B, Kraus JM, Frese KK, Kelkenberg S, Giese NA, Kestler HA, Gress TM, Buchholz M, PLAC8 Localizes to the Inner Plasma Membrane of Pancreatic Cancer Cells and Regulates Cell Growth and Disease Progression through Critical Cell-Cycle Regulatory Pathways, Cancer Res, 76 (2016) 96–107. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Ma H, Wang Y, Cao Z, Graves-Deal R, Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, Desai K, Gerdes MJ, Solnica-Krezel L, Coffey RJ, Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer, J. Clin. Invest, 124 (2014) 2172–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uehara H, Takahashi T, Izumi K, Induction of retinol-binding protein 4 and placenta-specific 8 expression in human prostate cancer cells remaining in bone following osteolytic tumor growth inhibition by osteoprotegerin, Int J Oncol, 43 (2013) 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsey C, Balakrishnan V, O’Dell MR, Huang JL, Newman L, Whitney-Miller CL, Hezel AF, Land H, Plac8 links oncogenic mutations to regulation of autophagy and is critical to pancreatic cancer progression, Cell Rep, 7 (2014) 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klionsky DJ, A human autophagy interaction network, Autophagy, 8 (2012) 439–441. [DOI] [PubMed] [Google Scholar]
- 26.Prasad SC, Thraves PJ, Dritschilo A, Rhim JS, Kuettel MR, Cytoskeletal changes during radiation-induced neoplastic transformation of human prostate epithelial cells, Scanning Microsc., 10 (1996) 1093–1102; discussion 1102–1094. [PubMed] [Google Scholar]
- 27.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS, Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18, Carcinogenesis, 18 (1997) 1215–1223. [DOI] [PubMed] [Google Scholar]
- 28.Achanzar WE, Diwan BA, Liu J, Quader ST, Webber MM, Waalkes MP, Cadmium-induced malignant transformation of human prostate epithelial cells, Cancer Res, 61 (2001) 455–458. [PubMed] [Google Scholar]
- 29.Achanzar WE, Webber MM, Waalkes MP, Altered apoptotic gene expression and acquired apoptotic resistance in cadmium-transformed human prostate epithelial cells, Prostate, 52 (2002) 236–244. [DOI] [PubMed] [Google Scholar]
- 30.Das TP, Suman S, Damodaran C, Induction of reactive oxygen species generation inhibits epithelial-mesenchymal transition and promotes growth arrest in prostate cancer cells, Mol Carcinog, 53 (2014) 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suman S, Das TP, Damodaran C, Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells, Br. J. Cancer, 109 (2013) 2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y, Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells, Cell Struct. Funct, 23 (1998) 33–42. [DOI] [PubMed] [Google Scholar]
- 33.Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC, Does bafilomycin A1 block the fusion of autophagosomes with lysosomes?, Autophagy, 4 (2008) 849–850. [DOI] [PubMed] [Google Scholar]
- 34.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB, Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma, J. Clin. Invest, 117 (2007) 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford ND, Shaw RJ, Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates, Mol. Cell, 59 (2015) 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM, mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin, Science, 341 (2013) 1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiarelli R, Roccheri MC, Heavy metals and metalloids as autophagy inducing agents: focus on cadmium and arsenic, Cells, 1 (2012) 597–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Yuan Y, Zou H, Yang J, Zhao S, Ma Y, Wang Y, Bian J, Liu X, Gu J, Liu Z, Zhu J, The ER stress regulator Bip mediates cadmium-induced autophagy and neuronal senescence, Sci Rep, 6 (2016) 38091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Son YO, Wang L, Poyil P, Budhraja A, Hitron JA, Zhang Z, Lee JC, Shi X, Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3beta/beta-catenin signaling, Toxicol Appl Pharmacol, 264 (2012) 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misra UK, Gawdi G, Pizzo SV, Induction of mitogenic signalling in the 1LN prostate cell line on exposure to submicromolar concentrations of cadmium+, Cell Signal, 15 (2003) 1059–1070. [DOI] [PubMed] [Google Scholar]
- 41.Jing Y, Liu LZ, Jiang Y, Zhu Y, Guo NL, Barnett J, Rojanasakul Y, Agani F, Jiang BH, Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells, Toxicol Sci, 125 (2012) 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu B, Chen S, Luo Y, Chen Z, Liu L, Zhou H, Chen W, Shen T, Han X, Chen L, Huang S, Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network, PLoS One, 6 (2011) e19052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watkin RD, Nawrot T, Potts RJ, Hart BA, Mechanisms regulating the cadmium-mediated suppression of Sp1 transcription factor activity in alveolar epithelial cells, Toxicology, 184 (2003) 157–178. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Ma W, Su Y, NF-kappaB pathway contributes to cadmium-induced apoptosis of porcine granulosa cells, Biol Trace Elem Res, 153 (2013) 403–410. [DOI] [PubMed] [Google Scholar]
- 45.Qu W, Ke H, Pi J, Broderick D, French JE, Webber MM, Waalkes MP, Acquisition of apoptotic resistance in cadmium-transformed human prostate epithelial cells: Bcl-2 overexpression blocks the activation of JNK signal transduction pathway, Environ. Health Perspect, 115 (2007) 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A, Life and death partners: apoptosis, autophagy and the cross-talk between them, Cell Death Differ, 16 (2009) 966–975. [DOI] [PubMed] [Google Scholar]
- 47.Pi H, Xu S, Reiter RJ, Guo P, Zhang L, Li Y, Li M, Cao Z, Tian L, Xie J, Zhang R, He M, Lu Y, Liu C, Duan W, Yu Z, Zhou Z, SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin, Autophagy, 11 (2015) 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Son YO, Wang X, Hitron JA, Zhang Z, Cheng S, Budhraja A, Ding S, Lee JC, Shi X, Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells, Toxicol Appl Pharmacol, 255 (2011) 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martens S, McMahon HT, Mechanisms of membrane fusion: disparate players and common principles, Nat Rev Mol Cell Biol, 9 (2008) 543–556. [DOI] [PubMed] [Google Scholar]


