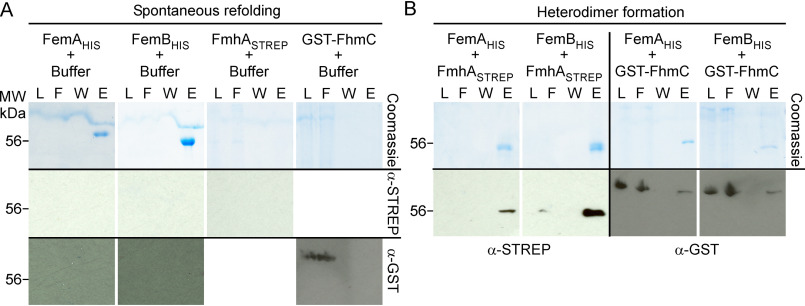
Figure 6.
Spontaneous refolding and heterodimer formation of Fem and Fmh proteins. A, purification over Ni-NTA following refolding of denatured proteins. Recombinant FemAHis, FemBHIS, FmhASTREP, and GST-FmhC were extracted from E. coli in the presence of 6 m guanidine hydrochloride. Protein preparations were diluted 10-fold in column buffer, spun at 100,000 × g to remove insoluble precipitates, and purified by gravity flow over Ni-NTA. B, heterodimer formation. Recombinant FmhASTREP and GST-FmhC prepared in 6 m guanidine hydrochloride were mixed in a pairwise fashion with either FemAHIS or FemBHIS prior to renaturation and purification over Ni-NTA as described above. Aliquots of the load (L), flow-through (F), wash (W) and eluates (E) collected during Ni-NTA purification were separated by SDS-PAGE and gels were stained with Coomassie Brilliant Blue or transferred to membranes for immunoblot analyses with α-STREP or α-GST antibodies. Numbers to the left of gels and blot indicate the position of the 56 kDa molecular weight (MW) marker.