Figure 2.
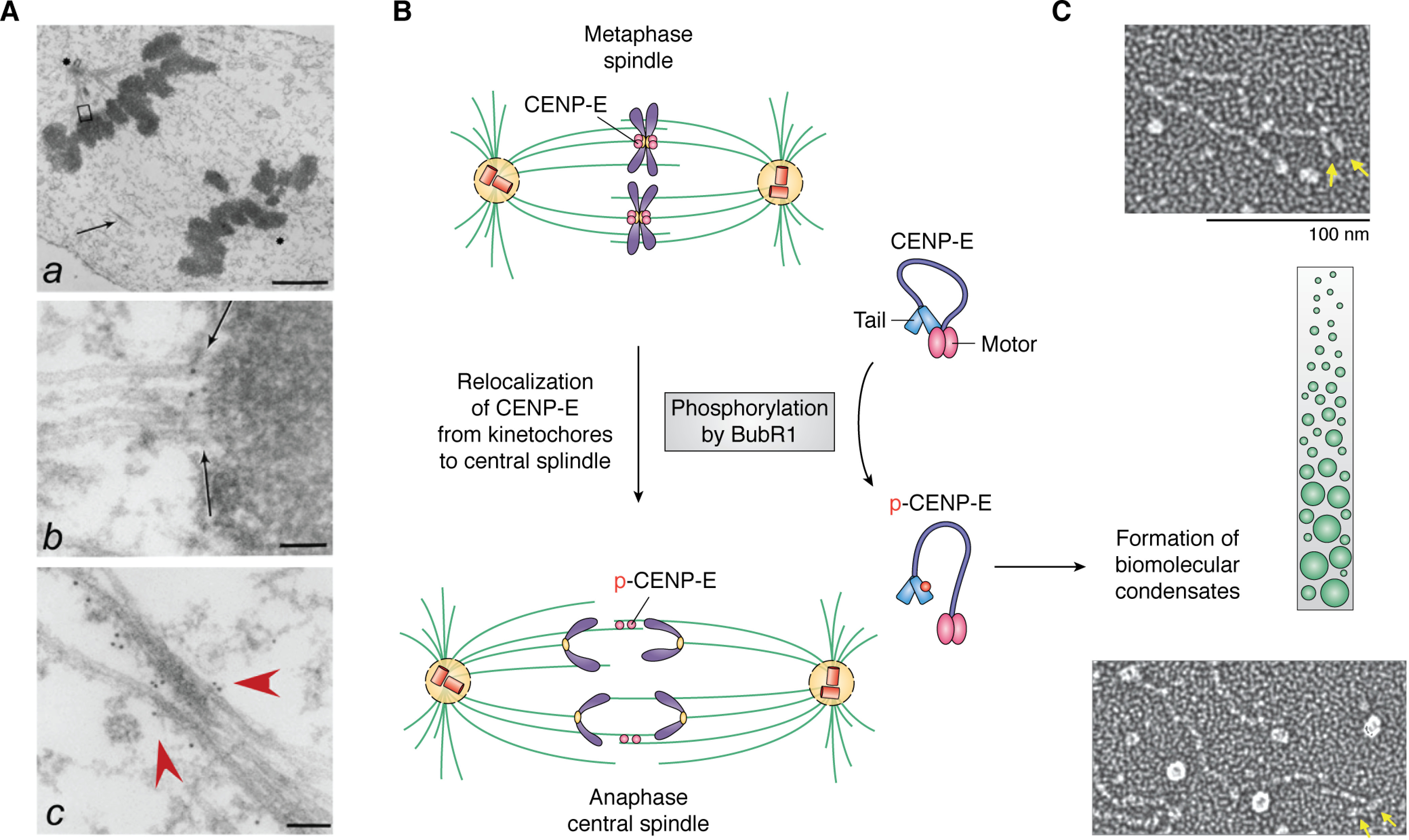
Membraneless organelle reorganization in mitosis is driven by LLPS and phosphorylation. A, electron microscopic image of an anaphase spindle showing membraneless organelles, including the centromere and the central spindle as well as the ultrastructure of chromatin. a, low-magnification view of a late anaphase HeLa cell showing elongated spindle poles, labeled with asterisks. One is apparent; another is in a different section. Interzonal microtubules are readily seen (arrow). b, magnified view of the upper boxed region in a, showing that CENP-E is located between a kinetochore and its associated spindle microtubules (arrow). c, magnified view of the area indicated by the arrow in a. Some CENP-E is now localized to the interzonal microtubules (red arrowheads). Bars, 2 μm (a), 70 nm (b), and 90 nm (c). 10-nm gold particles annotate the CENP-E molecules. Adapted from Ref. 68. B, schematic illustration of the reorganization of the metaphase spindle into an anaphase central spindle. Phosphorylation of CENP-E by BubR1 leads to intramolecular interactions of CENP-E and drives assembly of the central spindle. Unresolved questions are how LLPS drives coacervate formation and how BubR1 kinase activity is regulated in the coacervates. Adapted from Ref. 74. C, single-molecule electron microscopic analysis of CENP-E exhibiting its conformational changes. Adapted from Ref. 76. The green illustrates the crowding effect, whereas the yellow arrows indicate the motor domain of CENP-E. It remains to be examined whether BubR1 phosphorylation increases CENP-E molecule crowding during metaphase-anaphase transition.
