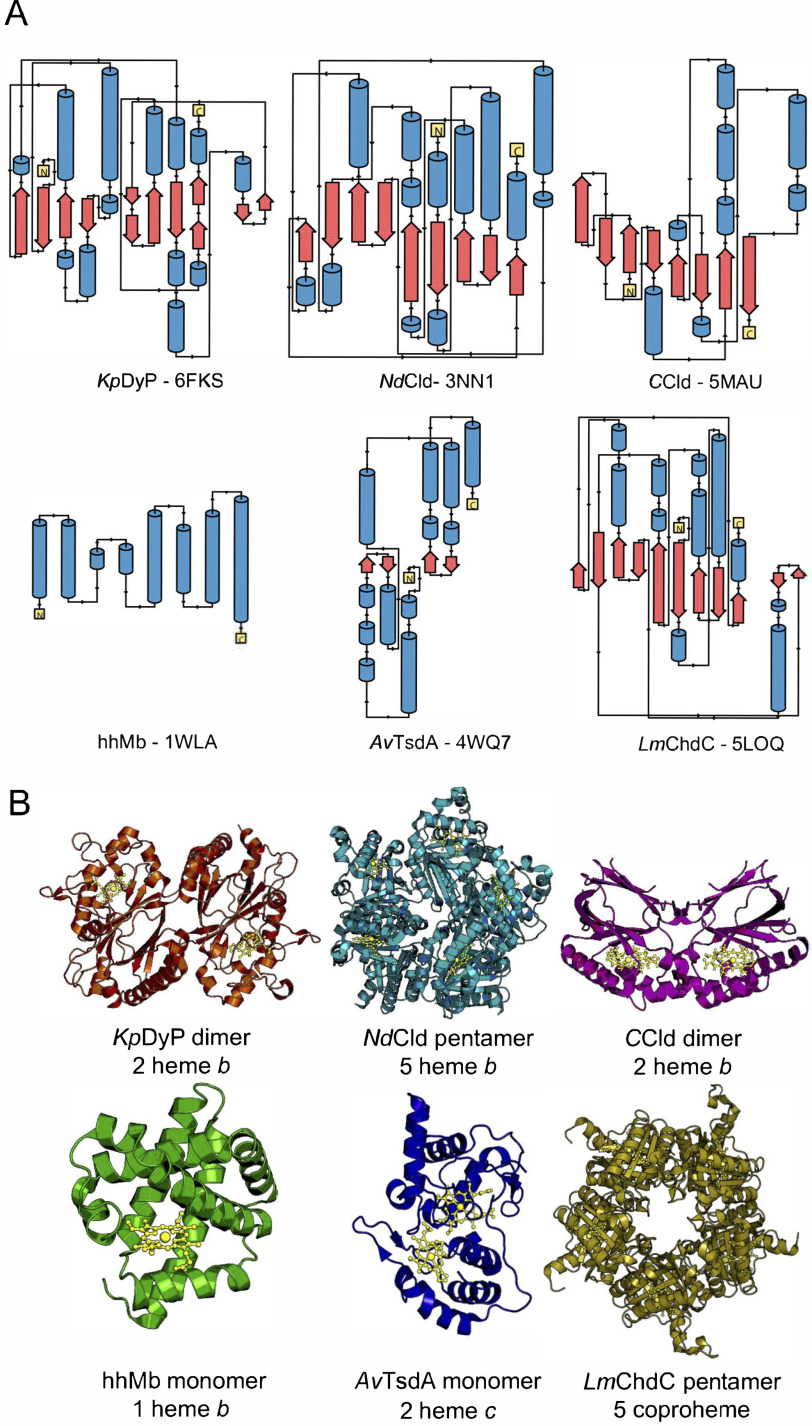
Figure 1.
Illustration of the diversity of secondary, tertiary and quaternary structure elements of the investigated heme proteins. A, secondary structure elements. α helices are shown in blue, β sheets are in red, and loop regions are shown as black lines. N and C termini are shown as yellow boxes. B, crystal structures of the investigated proteins. KpDyP (orange) and CCld (pink) form dimeric structures with one heme b moiety (gold) per subunit and a dimeric or truncated α-β ferredoxin fold. NdCld (turquoise) and LmChdC (yellow) form pentamers with one heme b or coproheme moiety per subunit and an α/β ferredoxin fold. hhMb (green) is a monomeric α-helical protein with one heme b cofactor. AvTsdA (blue) is a monomeric protein with two heme c cofactors and a predominantly α-helical structure. The protein backbone and cofactors are depicted as cartoon and ball-and-stick representations, respectively.