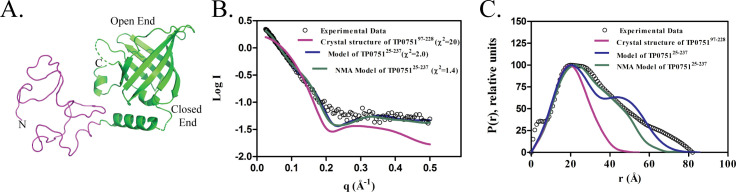
Fig 1. SAXS analysis of full-length TP0751.
(A) Ribbon diagram of the bipartite structural model of full-length TP0751 generated using the intensive mode of Phyre2 [56]. The unstructured N-terminal region (residues 25–96) and C-terminal lipocalin domain (residues 97–237) are shown in pink and green, respectively. (B) Plots showing the log of the scattering intensity (I) as a function of momentum transfer (q = 4πsin(θ)/λ). The black circles are SAXS experimental data; the colored curves are theoretical scattering profiles calculated from different structural models using FoXS [123]. The χ2 shown in parentheses indicate the fit of the theoretical scatterings to the experimental SAXS data. (C) Comparison of the normalized inter-atomic pairwise distribution function (P(r)), computed from the experimental SAXS data (black circles) and different 3D models (colored lines). The P(r) functions show that TP075125-237 has a bipartite architecture with a diameter of approximately 75Å.