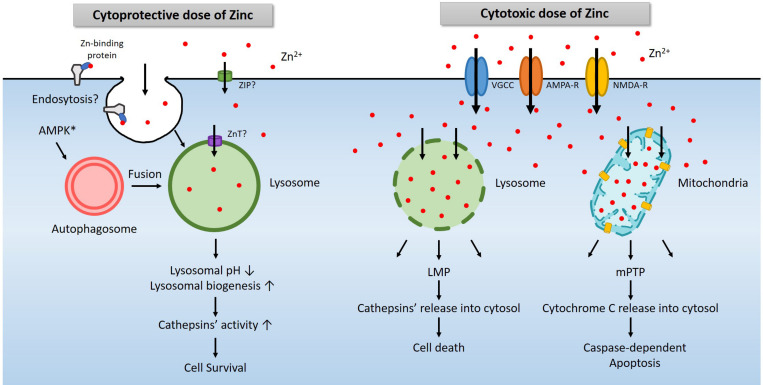
FIGURE 2.
A diagram for the role of lysosomes in zinc-related cell survival and death. Under physiological conditions, a modest increase in cytosolic free zinc translates into a modest increase in lysosomal free zinc due to the function of zinc transporters (Lichten and Cousins, 2009). In addition, endocytosis of zinc-binding proteins also increases lysosomal zinc levels (Rowe and Bobilya, 2000). AMPK contributes to the activation of lysosome via the autophagy pathway (Young et al., 2016; Jang et al., 2018). An increase of free zinc in the lysosome induces lysosomal acidification and activates lysosomal enzymes such as cathepsins. In most cases, these changes promote cell survival (Park et al., 2011; Seo et al., 2015; Lee et al., 2017). However, a high concentration of extracellular zinc enters the cytosol through voltage-gated calcium channel (VGCC), calcium-permeable AMPA receptor (AMPA-R), or NMDA-R, and then lysosomes or mitochondria likely via zinc transporters (Sensi and Jeng, 2004). Excessive zinc in lysosome or mitochondria leads to LMP (Hwang et al., 2008) and mPTP (Wudarczyk et al., 1999; Jiang et al., 2001), which releases cathepsins and other lysosomal enzymes or cytochrome C to causes cell death (Hwang et al., 2008; Mrschtik and Ryan, 2015).