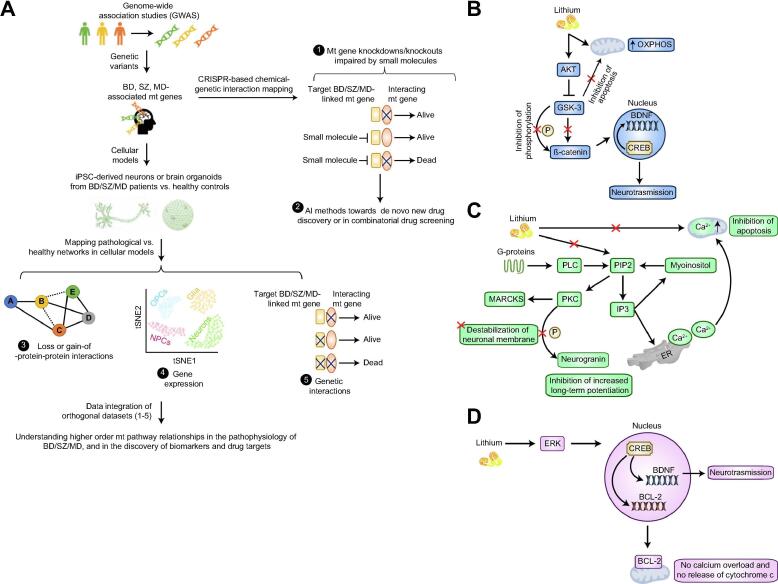
Fig. 1.
Conceptual systems framework in NPDs (BD/SZ/MD), and lithium treatment amelioration of mitochondrial (mt) functions in distinct pathways. (A) Genetic variants from GWAS will allow the discovery of NPD-associated mt risk genes. A CRISPR-based chemical-genetic interaction screening can unveil NPD-relevant mt targets inhibited by a small molecule. Here, knockouts (or knockdowns) of the interacting gene of NPD-associated mt risk factors is lethal when cells are treated with small molecule. Artificial intelligence (AI) methods, such as deep learning can be employed towards de novo drug discovery and combinatorial drug screening. By utilizing iPSC-derived neurons or 3D brain organoids from NPD patients vs. healthy subjects, loss (dotted lines)-or gain (thick lines) of protein–protein interactions (PPIs), gene expression in distinct cell states (OPC, oligodendrocytes; NPCs, Neural progenitor cells), and genetic interaction networks (i.e. two functionally linked single gene knockouts show lethal phenotype) can be mapped to pinpoint specific or common pathological macromolecular complexes (identified from PPI networks) or pathways involved in the pathophysiology of BD/SZ/MD. Integration of PPIs with co-expression profiles from scRNA-seq should uncover functional modules and driver genes of NPDs. Notably, a gene knockout that show chemical-genetic interaction with a small molecule should display lethal with a mutation in the drug target gene. Thus, comparing genetic interactions with chemical-genetic interaction profiles will identify mt pathway targets modulated by small molecule. Collectively, integration of these orthogonal datasets in various combinations should generate a higher order pathway-level understanding of NPD-related mt genes and in the discovery of disease biomarkers and drug targets. (B) Lithium inhibits GSK-3 pathway, allowing the translocation of β-catenin to the nucleus and transcription of pro-survival factors. Lithium prevents mt translocation of GSK-3 and improves oxidative phosphorylation (OXPHOS). (C) Lithium impedes the phosphoinositol cycle, reducing myoinositol levels and, consequently, blocking the activation of PKC pathway. Inhibition of this pathway prevents destabilization of neuronal membranes, increased long-term potentiation, and induction of apoptosis. (D) BCL-2 and BDNF factors are improved via ERK activation. Increased expression of these two proteins ameliorates neurotransmission, avoids mt Ca2+ overload and prevents cytochrome c release.