Summary
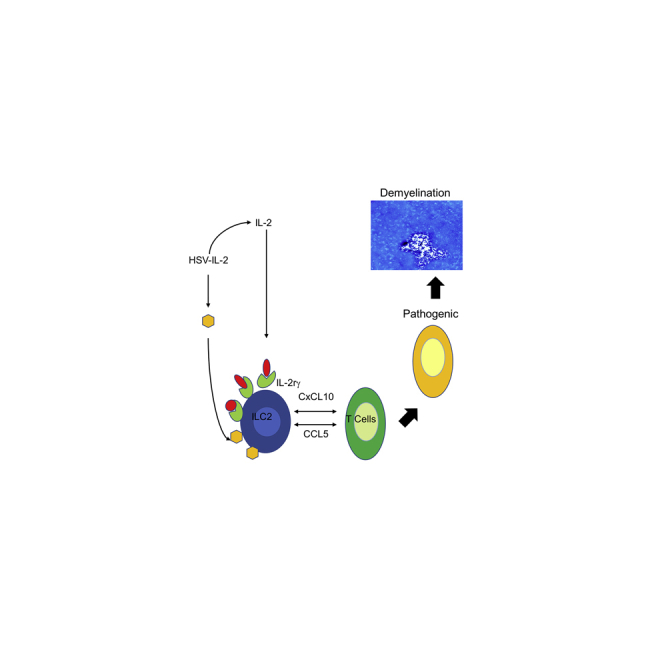
We previously reported that infection of different mouse strains with a recombinant HSV-1 expressing IL-2 (HSV-IL-2) caused CNS demyelination. Histologic examination of infected IL-2rα−/−, IL-2rβ−/−, and IL-2rγ−/− mice showed demyelination in the CNS of IL-2rα−/− and IL-2rβ−/− mice but not in the CNS of IL-2rγ−/−-infected mice. No demyelination was detected in mice infected with control virus. IL-2rγ−/− mice that lack type 2 innate lymphoid cells (ILC2s) and ILCs, play important roles in host defense and inflammation. We next infected ILC1−/−, ILC2−/−, and ILC3−/− mice with HSV-IL-2 or wild-type (WT) HSV-1. In contrast to ILC1−/− and ILC3−/− mice, no demyelination was detected in the CNS of ILC2−/−-sinfected mice. However, transfer of ILC2s from WT mice to ILC2−/− mice restored demyelination in infected recipient mice. CNS demyelination correlated with downregulation of CCL5 and CXCL10. This study demonstrates that ILC2s contribute to HSV-IL-2-induced CNS demyelination in a mouse model of multiple sclerosis.
Subject Areas: Immunology, Neuroscience
Graphical Abstract
Highlights
-
•
IL-2rγ−/−, but not IL-2rα−/− or IL-2rβ−/−, mice are protected from CNS demyelination
-
•
Mice lacking ILC2s, but not ILC1s or ILC3s, are protected from CNS demyelination
-
•
Transfer of ILC2s from WT to ILC2−/− mice restore CNS demyelination to infected mice
-
•
Suppression of CCL5 and CXCL10 correlated with CNS demyelination
Immunology; Neuroscience
Introduction
Degradation of the myelin sheath in the brain, optic nerve (ON), and spinal cord has been associated with a number of diseases, with multiple sclerosis (MS) being the most common syndrome of CNS inflammatory demyelination (Hunter et al., 1997; Martin et al., 1992; Noseworthy et al., 2000; Sospedra and Martin, 2005). The World Health Organization estimates that over 2.5 million people suffer from MS globally and according to the National MS Society, approximately 400,000 Americans have MS. The economic impact of MS in the US is estimated to be more than $28 billion per year, and available therapies are generally not effective for MS. Epidemiologic studies have implicated environmental and genetic factors in the development of MS, and it has been suggested that infectious agents (Hafler, 2004; Hemmer et al., 2002), particularly certain viruses, may be involved in this process (Challoner et al., 1995; Friedman et al., 1999). However, this concept remains controversial (Boman et al., 2000; Martin et al., 1997; Mirandola et al., 1999) and, if an infectious agent is involved, it may not be sufficient to initiate the disease.
Several lines of evidence suggest that the cytokine IL-2 is involved in demyelination during MS progression. First, the number of IL-2-secreting cells and amount of IL-2 in the sera of MS patients are elevated (Gallo et al., 1988, 1989; Lu et al., 1993; Trotter et al., 1989), and second, levels of soluble IL-2 receptor (sIL-2r) are increased in both the sera (Bansil et al., 1991; Gallo et al., 1989; Greenberg et al., 1988; Hartung et al., 1990; Traugott, 1987) and CSF of MS patients (Adachi et al., 1989; Kittur et al., 1990). In addition, supernatants from MS patients' T lymphocytes cause damage to myelin and glial cells in vitro (Selmaj et al., 1988a, b), suggesting that MS T lymphocytes are pre-activated in vivo to produce demyelination factors. In these studies, the percentage of MS patient T lymphocytes that express the IL-2r correlates with the degree of supernatant-induced demyelination in vitro. The presence of IL-2 is also associated with disease state in MS mouse models (McCombe et al., 1998; Petitto et al., 2000; Yang et al., 2002). To dissect the role of IL-2 in the context of viral infection during MS, we constructed a recombinant herpes simplex virus type 1 (HSV-1) that constitutively expresses mouse IL-2 (HSV-IL-2) and constructed similar recombinant viruses expressing mouse IL-4, IFN-γ, IL-12p35, or IL12p40 genes for use as controls (Ghiasi et al., 2002b; Osorio and Ghiasi, 2003; Osorio et al., 2003; Zandian et al., 2009). Mice that were ocularly infected with HSV-IL-2 developed optic neuropathy as determined by changes in visual-evoked cortical potentials (VECPs) (Zandian et al., 2009) and pathologic changes in the ON and CNS (Osorio et al., 2005; Zandian et al., 2009), whereas recombinant HSV viruses expressing IL-4, IFN-γ, IL-12p35, IL-12p40, or IL-12p70 did not induce optic neuropathy or CNS pathology (Ghiasi et al., 2001, 2002a). Similarly, delivery of IL-2 into the brains of mice using Alzet osmotic mini-pumps prior to ocular infection with wild-type (WT) HSV-1 produced eye disease and CNS pathology whereas WT HSV-1 alone did not, nor did injection of IL-2 DNA, IL-2 protein, or IL-2 peptides into other mouse strains prior to infection with wt HSV-1 (Mott et al., 2013).
IL-2 is a pleiotropic cytokine that plays a major role in regulating the adaptive immune response (Waldmann, 2006). IL-2 signals through its heterotrimeric receptor consisting of α (IL-2rα, CD25), β (IL-2rβ, CD122), and γ (IL-2rγ, CD132) chains (Minami et al., 1993; Waldmann, 2006). IL-2rα is the low-affinity IL-2r expressed on activated T and B lymphocytes. IL-2rα alone is not a signaling receptor and pairs with IL-2rβ chain (expressed constitutively on a subset of CD8+ spleen T cells, on NK cells, and at lower levels on a small population of resting B cells) or IL-2rγ (expressed constitutively at low levels on most lymphocytes, myeloid cells, and embryonic thymocytes) (Minami et al., 1993; Waldmann, 2006). In addition to IL-2, IL-2rγ is also the receptor for IL-4, IL-7, IL-9, IL-15, and IL-21 (Waldmann, 2006).
Our published studies showing that elevated IL-2 levels, together with an environmental factor—viral infection—can initiate CNS demyelination are consistent with other published studies. Indeed, published studies on the function of IL-2 in CNS demyelination suggest that its atypical activation is linked to pathogenesis. Whether IL-2 can directly, or by binding to individual, or combinations of its receptors, contribute to CNS demyelination is not known. To determine the role of IL-2rs in CNS demyelination we ocularly infected IL-2rα−/−, IL-2rβ−/−, and IL-2rγ−/− mice with HSV-IL-2 recombinant virus and control WT virus. No demyelination was detected in IL-2rγ−/−-infected mice, implicating a role for innate lymphoid cells (ILCs) in CNS demyelination. Ocular infection of ILC1−/−, ILC2−/−, and ILC3−/− mice with HSV-IL-2 suggested that ILC2s play a role in HSV-IL-2-induced CNS demyelination. Adoptive transfer of bone-marrow-derived ILC2s from WT mice to ILC2−/− mice restored demyelination in recipient mice. Our results demonstrate that ILC2s do play a significant role in determining the outcomes of CNS demyelination following ocular HSV-IL-2 infection in mice.
Results
Role of IL-2rs in HSV-IL-2-Induced Demyelination in Infected Mice
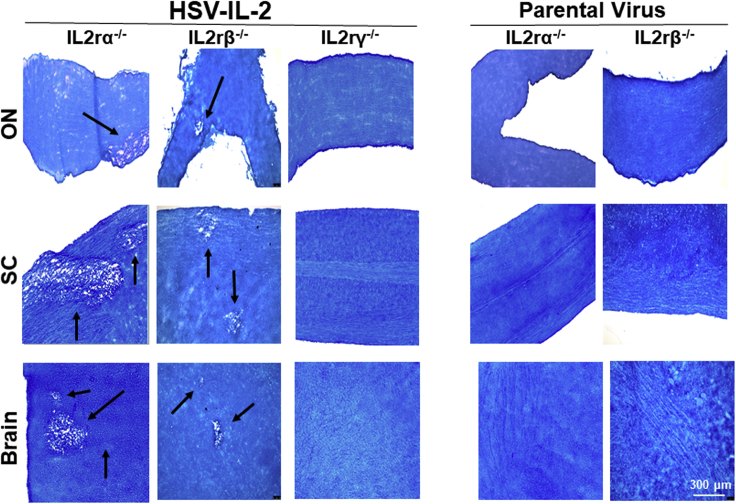
IL-2 binds to the IL-2 receptor (IL-2r), which has three forms: α (i.e. IL-2rα or CD25), β (i.e. IL-2rβ or CD122), and γ (i.e. IL-2rγ or CD132). A functional IL-2 receptor is generated by homo- or heterogeneous combinations of these forms having different affinities on different cell types (Minami et al., 1993; Waldmann, 2006). To determine the possible involvement of IL-2rs in regulating HSV-IL-2-induced demyelination, we infected IL-2rα−/−, IL-2rβ−/−, and IL-2rγ−/− mice ocularly with HSV-IL-2 as we described previously (Osorio et al., 2005; Zandian et al., 2009; Mott et al., 2015). Control mice were similarly infected with parental virus. We previously reported that CNS demyelination is observed after day 10 postinfection (PI) with HSV-IL-2 virus (Zandian et al., 2009). Thus, at day 14 PI, mice in the current study were sacrificed and brain, spinal cord, and ON were collected, postfixed, and stained with the myelin stain, Luxol Fast Blue (LFB), as we described previously (Osorio et al., 2005; Zandian et al., 2009). Representative photomicrographs of brain, spinal cord, and ON sections from mice infected with HSV-IL-2 or parental virus are shown in Figure 1.
Figure 1.
Role of IL-2 Receptors in HSV-IL-2-Induced CNS Demyelination
Female IL-2rα−/−, IL-2rβ−/−, and IL-2rγ−/− mice were infected ocularly with HSV-IL-2 or parental virus as described in Transparent Methods. On day 14 PI, brain, spinal cord, and ON were collected, fixed, sectioned, and stained with LFB. Representative photomicrographs are shown. Arrows indicate areas of demyelination. ×10 objective lens was used.
Demyelination was observed in brain, spinal cord, and ON of IL-2rα−/− and IL-2rβ−/− mice infected with HSV-IL-2 (Figure 1, HSV-IL-2 panels), whereas no demyelination was detected in brain, spinal cord, and ON of IL-2rα−/− and IL-2rβ−/− mice infected with parental HSV (Figure 1, Parental panels). In contrast, no demyelination was detected in brain, spinal cord, and ON of IL-2rγ−/− mice infected with HSV-IL-2 (Figure 1A, HSV-IL-2 panels). Thus, our results suggest that the absence of IL-2rγ blocks HSV-IL-2-induced CNS demyelination in ocularly infected mice, whereas the absence of IL-2rα and IL-2rβ does not.
Role of ILCs in HSV-IL-2-Induced Demyelination in Infected Mice
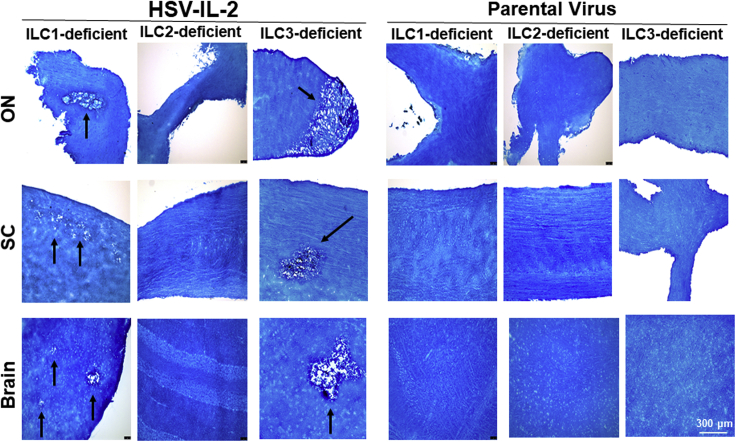
The above results suggest that the absence of IL-2rγ, but not the absence of IL-2rα and IL-2rβ, blocked HSV-IL-2-induced CNS demyelination. Previously it was reported that CD132−/− mice lack ILC2s (Wong et al., 2012; Yokota et al., 1999). IL-2 is a critical regulator of ILC2 function (Roediger et al., 2015). In addition to ILC2s, CD132−/− mice also are lacking NK cells (Cao et al., 1995). ILCs are known to play important roles in host defense, metabolic homeostasis, and tissue repair. Increasing evidence that ILCs contribute to inflammation is stimulating interest in developing therapeutic strategies to target specific ILC populations (Abt et al., 2016a, 2016b; McKenzie et al., 2014; Seillet et al., 2016; Rafei-Shamsabadi et al., 2018, Zook and Kee, 2016). Three major ILC members are ILC1s, ILC2s, and ILC3s (Spits et al., 2013). To determine the contribution of ILC2s to CNS demyelination and if ILC1s or ILC3s also have a role in HSV-IL-2-induced CNS demyelination, we ocularly infected ILC1−/−, ILC2−/−, and ILC3−/− mice with HSV-IL-2 as in Figure 1. Control mice were similarly infected with parental virus. At day 14 PI, mice were sacrificed and brain, spinal cord, and ONs were collected, post-fixed, and stained with the myelin stain, LFB, as described earlier. Photomicrographs of brain, spinal cord, and ON sections from mice infected with HSV-IL-2 or parental virus are shown in Figure 2. Demyelination was detected in brain, spinal cord, and ON of ILC1−/− and ILC3−/− mice but not in ILC2−/− mice (Figure 2, HSV-IL-2 panels). As expected, no demyelination was detected in brain, spinal cord, and ON of ILC1−/−, ILC2−/−, and ILC3−/− mice infected with parental virus (Figure 2, parental virus). These results suggest that the absence of demyelination in IL-2rγ−/− mice is due to the absence of ILC2s and not natural killer (NK) cells.
Figure 2.
Role of ILCs in HSV-IL-2-Induced CNS Demyelination
Female ILC1−/−, ILC2−/−, and ILC3−/− mice were infected ocularly with HSV-IL-2 or parental virus as described in Transparent Methods. On day 14 PI, brain, spinal cord, and ON were collected, fixed, sectioned, and stained with LFB. Representative photomicrographs are shown. Arrows indicate areas of demyelination. ×10 objective lens was used.
Role of ILC2s in HSV-IL-2-Induced CNS Demyelination
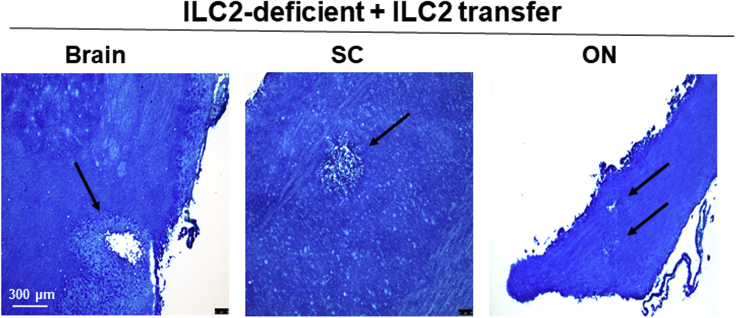
Because the above results suggested that ILC2s are involved in CNS demyelination (Figure 2), ILC2s were isolated from bone marrow of naive WT mice, and the cells were injected intravenously into ILC2−/− recipient mice as described in Transparent Methods. Two weeks after adoptive transfer, all ILC2−/− recipient mice were infected ocularly with HSV-IL-2. Fourteen days after infection, the mice were sacrificed and brain, spinal cord, and ON were removed, post-fixed, and stained with LFB. Representative photomicrographs are shown in Figure 3. We found that adoptive transfer of ILC2s caused demyelination in brain, spinal cord, and ON of ILC2−/− recipient mice (Figure 3). Patterns of demyelination in the brain, SC, and ON of ILC2−/− recipient mice was similar to that of WT mice. The results of these studies provide further evidence that ILC2s contribute to CNS demyelination.
Figure 3.
Demyelination in ILC2−/− Mice Following Adoptive Transfer of ILC2s
Bone-marrow-derived ILC2s were isolated from naive WT mice and transferred IV into recipient ILC2−/− mice. Fourteen days postadoptive transfer, recipient ILC2−/− mice were infected ocularly with HSV-IL-2. On day 14 PI, brain, spinal cord, and ON were collected, fixed, sectioned, and stained with LFB. Representative photomicrographs are shown. Arrows indicate areas of demyelination. ×10 objective lens was used.
Virus-Expressed IL-2 Binds to the Cell Surface of ILC2s In Vitro
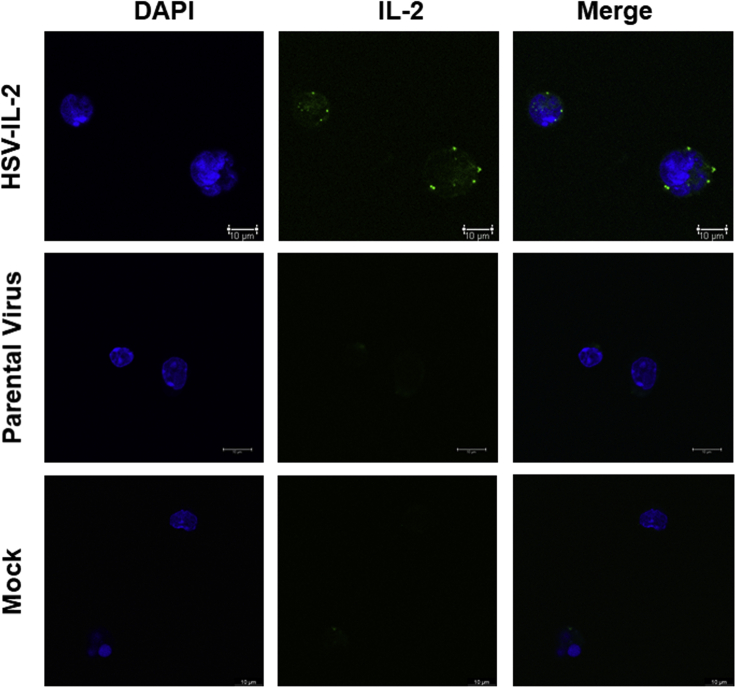
To determine if IL-2 expressed by HSV-IL-2 binds to ILC2s, isolated ILC2s were infected with HSV-IL-2 or parental virus or mock infected as described in Transparent Methods. The cells were stained with anti-IL-2 antibody and analyzed using confocal microscopy. The results verified that IL-2 expressed by HSV-IL-2 was detected on the surface of infected ILC2s, whereas no IL-2 expression was detected in parental- or mock-infected cells Figure 4). Similar to our previous studies (Ghiasi et al., 2002b; Osorio et al., 2005), this result shows that IL-2 is expressed in infected ILC2s and also binds to the surface of purified ILC2s.
Figure 4.
IL-2 Expressed by HSV-IL-2 UL20 Colocalizes with Infected ILC2s. ILC2s were Infected with 10 PFU/Cell of HSV-IL-2, Parental Virus, or Mock Infected
Infection was allowed to proceed for 24 h and then the slides were fixed, blocked, and stained with anti-IL-2 (green) antibody and DAPI nuclear stain (blue). Mock-infected cells were treated similarly and used as controls. Images were acquired using confocal microscopy. Photomicrographs are shown at ×630 total magnification. ×63 objective lens was used.
Identifying Genes that may Contribute to CNS Demyelination in WT Mice
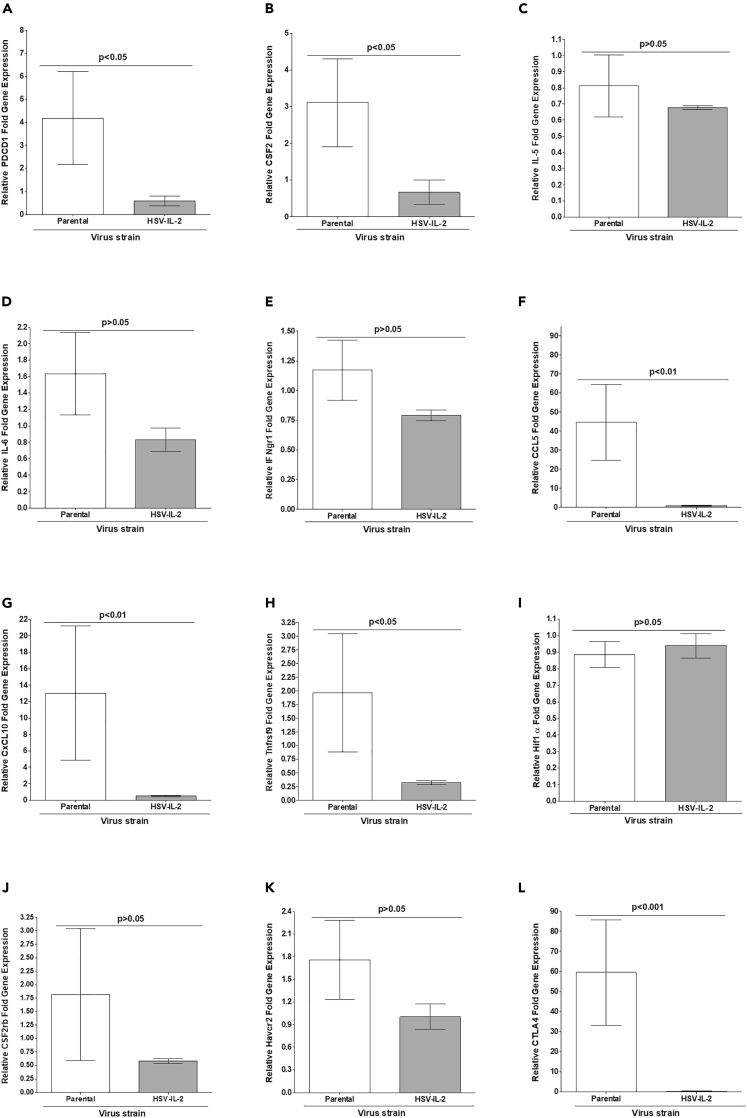
HSV-IL-2 infection of WT mice causes CNS demyelination, whereas infection of mice with parental virus does not (Dumitrascu et al., 2014; Mott et al., 2013; Zandian et al., 2009, 2011a, 2011b). Based on MS and experimental autoimmune encephalomyelitis (EAE) published studies, we investigated the roles of selected Pdcd1 (PD1), CSF2 (GM-CSF), IL-5, IL-6, IFNgr1, CCL5 (RANTES), CXCL10, Tnfrsf9 (4-1BB/CD137), HIF1α, CSF2rb, Havcr2 (Tim-3), and CTLA4 genes in CNS demyelination in vivo. On day 14 PI, RNAs were isolated as previoulsy described (Mott et al., 2007a, Mott et al., 2007b) from brains of WT mice infected with HSV-IL-2 or parental virus. The results are presented in Figure 5 as “fold increase” in WT mice infected with HSV-IL-2 or parental virus compared with the baseline mRNA levels in brains of WT uninfected naive mice. Levels of PD1 (Figure 5A; p < 0.05), GM-CSF (Figure 5B; p < 0.05), CCL5 (Figure 5F; p < 0.01), CCXL10 (Figure 5G; p < 0.01), 4-1BB (Figure 5H; p < 0.05), and CTLA4 (Figure 5L; p < 0.001) were significantly lower in HSV-IL-2-infected mice than in parental-virus-infected mice. In contrast, levels of IL-5 (Figure 5C; p > 0.05), IL-6 (Figure 5D; p > 0.05), IFNgr1 (Figure 5E; p > 0.05), HIF1α (Figure 5I; p > 0.05), CSF2rb (Figure 5J; p > 0.05), and Tim-3 (Figure 5K; p > 0.05) were similar in HSV-IL-2-infected and parental-infected mice.
Figure 5.
Identifying Genes that may Contribute to CNS Demyelination in WT Mice
Effects of viral IL-2 on gene expression in the brain of HSV-1-infected WT mice were determined on day 14 PI. RNA isolated from brains of WT mice infected with HSV-IL-2 or parental virus was used to measure the expression of Pdcd1 (PD1), CSF2 (GM-CSF), IL-5, IL-6, IFNgr1, CCL5 (RANTES), CXCL10, Tnfrsf9 (4-1BB/CD137), HIF1α, CSF2rb, Havcr2 (Tim-3), and CTLA4 in virus-infected brains. qRT-PCR was performed using total RNA as described in the Transparent Methods. Expression of respective genes in naive WT mice was used as a baseline control to estimate relative expression of each transcript in brains of infected mice. GAPDH expression was used to normalize the relative expression of each transcript. Each point represents the mean ± SEM from three brains for each mouse strain. p values were determined using a one-way ANOVA test.
Panels: (A) PD1 transcript; (B) CSF2 (GM-CSF) transcript; (C) IL-5 transcript; (D) IL-6 transcript; (E) IFNgr1 transcript; (F) CCL5 (RANTES) transcript; (G) CXCL10 transcript; (H) Tnfrsf9 (4-1BB/CD137) transcript; (I) HIF1α transcript; (J) CSF2rb transcript; (K) Havcr2 (Tim-3) transcript; and (L) CTLA4 transcript.
Identifying Genes that may Contribute to CNS Demyelination in ILC2−/− Mice
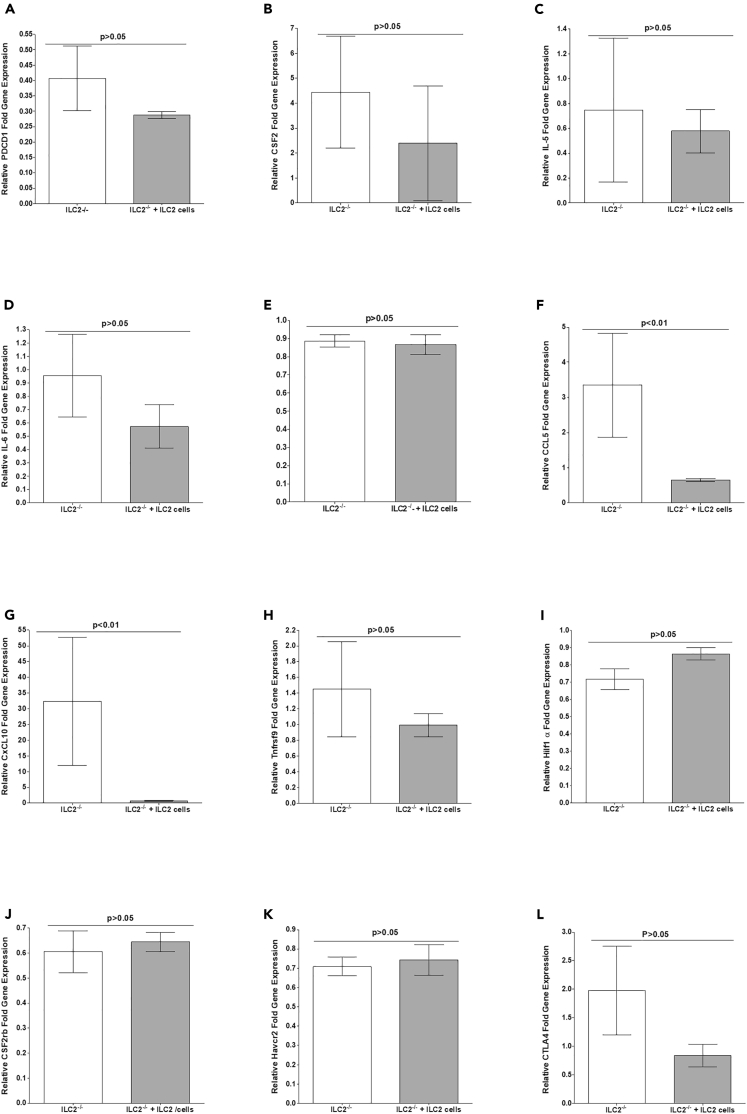
The above results suggest that the absence of ILC2 in ILC2-deficient mice blocked CNS demyelination following infection of ILC2−/− mice with HSV-IL-2 virus, whereas restoring ILC2 by transferring ILC2 to ILC2-deficient mice restored CNS demyelination in HSV-IL-2-infected mice (Figures 2 and 3). To determine the contribution of ILC2 to CNS demyelination, we compared gene expression in mouse brains from ILC2-deficient and ILC2-restored mice following infection with HSV-IL-2 virus as in Figure 5. RNAs were isolated from brains of virus-infected ILC2-deficient and ILC2-restored mice, and relative levels of the same 12 transcripts shown in Figure 5 were determined by RT-qPCR. “Fold increase” in ILC2-deficient and ILC2-restored mice infected with HSV-IL-2 virus was compared with baseline mRNA levels in brains of uninfected naive ILC2-deficient mice (Figure 6). Levels of CCL5 (Figure 6F; p < 0.01) and CXCL10 (Figure 6G; p < 0.01) were significantly lower in ILC2-transferred mice, which is consistent with results obtained with WT mice infected with HSV-IL-2. However, we did not detect significant differences in PD1 (Figure 6A, p > 0.05), GM-CSF (Figure 6B, p > 0.05), IL-5 (Figure 6C; p > 0.05), IL-6 (Figure 6D; p > 0.05), IFNgr1 (Figure 6E; p > 0.05), 4-1BB (Figure 6H; p > 0.05), HIF1α (Figure 6I; p > 0.05), CSF2rb (Figure 6J; p > 0.05), Tim-3 (Figure 6K; p > 0.05), and CTLA4 (Figure 6L, p > 0.05) mRNA levels between ILC2-deficient and ILC2-transferred mice. Thus, similar to WT mice, upregulation of CCL5 and CXCL10 correlates with CNS demyelination upon infection. However, results for PD1, GM-CSF, 4-1BB, and CTLA4 differed between WT and ILC2−/− infected mice.
Figure 6.
Determining Genes that may Contribute to CNS Demyelination in ILC2−/− Mice
LC2-deficient mice or ILC2-deficient mice that received ILC2 from WT mice were infected with HSV1-IL-2. On day 14 PI, RNA was isolated from brains of ILC2-deficient mice or ILC2-deficient mice that received ILC2 from WT as described in Figure 3. qRT-PCR was performed using total RNA, and expression of respective genes in naive ILC2-deficient mice was used as a baseline to estimate relative expression of each transcript in brains of virus-infected mice. GAPDH expression was used to normalize the relative expression of each transcript. Each point represents the mean ± SEM from three brains for each group of mice. p value was determined using a one-way ANOVA test.
Panels: (A) PD1 transcript; (B) CSF2 (GM-CSF) transcript; (C) IL-5 transcript; (D) IL-6 transcript; (E) IFNgr1 transcript; (F) CCL5 (RANTES) transcript; (G) CXCL10 transcript; (H) Tnfrsf9 (4-1BB/CD137) transcript; (I) HIF1α transcript; (J) CSF2rb transcript; (K) Havcr2 (Tim-3) transcript; and (L) CTLA4 transcript.
Discussion
Epidemiologic studies have implicated both environmental and genetic factors in MS (Hunter et al., 1997; Noseworthy et al., 2000). MS is an autoimmune disease, perhaps initiated by a viral infection, that attacks and degrades the myelin sheath (Martin et al., 1992). Numerous viruses have been proposed as causative agents. Various herpes viruses, including HSV-1, HSV-2, HCMV, EBV, HHV-6, and HHV-7, have been implicated as the trigger for an autoimmune response leading to MS (Daibata et al., 2000; Ferrante et al., 2000; Knox et al., 2000), although other studies have disputed these findings (Nicoll et al., 1992; Taus et al., 2000). EAE is the primary experimental animal model for MS (Zamvil and Steinman, 1990). However, outcomes in the EAE model are influenced by the species and strain of experimental animals, materials used for immunization, and the type of adjuvant employed (Martin et al., 1992). In addition to the EAE model of MS, numerous other animal models for MS that have been developed generally use either the viral model (Bureau et al., 1998) or the direct autoimmune model (Cua et al., 1999) to initiate disease. Because IL-2 has been implicated in MS (Gallo et al., 1988, 1989; Lu et al., 1993; Trotter et al., 1989) and viruses have been implicated in initiating MS (Daibata et al., 2000; Ferrante et al., 2000; Knox et al., 2000), we explored the effects of infecting mice with a recombinant HSV-1 that expresses murine interleukin-2 (HSV-IL-2) (Ghiasi et al., 2002b). In contrast to other models of demyelination, this HSV-IL-2 model of CNS demyelination incorporates both a viral (i.e., HSV-1) and an immune component (i.e., IL-2). Using our HSV-IL-2 model of CNS demyelination we have made the following nine observations: (1) Ocular infection of female BALB/c, C57BL/6, SJL/6, and 129SVE mice with HSV-IL-2 results in demyelination in the brain, spinal cord, and ONs of infected mice (Osorio et al., 2005; Zandian et al., 2009). (2) Ocular infection with parental or WT viruses, or with similarly constructed recombinant HSV-1 expressing either IFN-γ (HSV- IFN-γ) or IL-4 (HSV-IL-4) does not induce CNS demyelination (Osorio et al., 2005). (3) Similar to the MS condition, female mice were more susceptible to HSV-IL-2-induced demyelination than were male mice (Zandian et al., 2009). (4) We detected CNS demyelination after delivering IL-2 into the mouse brain using osmotic mini-pumps or by injecting mice with rIL-2 protein, IL-2 DNA, or IL-2 synthetic peptides prior to infection with the WT HSV-1 strains McKrae and KOS (Mott et al., 2013). (5) A single mutation in the IL-2 open reading frame (T27A) completely blocked CNS demyelination in this model (Mott et al., 2013). (6) CD4+ and CD8+ T cells are both involved in HSV-IL-2-induced CNS demyelination, whereas macrophages are protective (Mott et al., 2011; Zandian et al., 2011a); (7) DCs, NK cells, and B cells play no role in demyelination (Zandian et al., 2011a). (8) HSV-IL-2-induced CNS demyelination was blocked by co-infecting mice with a recombinant HSV-1 expressing IL-12p70 (HSV-IL-12p70) or injecting with IL-12p70 DNA (Mott et al., 2011). Lastly, (9) a comparison of MOG35–55, MBP35–47, and PLP190–209 models of EAE with our HSV-IL-2-induced MS model (Dumitrascu et al., 2014) showed that our HSV-IL-2 model was similar to the MOG model and both differed from the MBP and PLP models.
ILC1s, ILC2s, and ILC3s have been shown to play important roles in host defense, metabolic homeostasis, tissue repair, and can contribute to inflammation (Abt et al., 2016a, 2016b; McKenzie et al., 2014; Seillet et al., 2016; Zook and Kee, 2016). Previous studies found that IL-2 signaling pathway plays an important role in regulating ILC2s (Minami et al., 1993; Roediger et al., 2015; Waldmann, 2006). In the current study, we evaluated the role of IL-2 receptors (IL-2rα, IL-2rβ, and IL-2rγ) and types 1, 2, and 3 ILCs in HSV-IL-2-induced CNS demyelination. We detected demyelination plaques in brain, spinal cord, and ONs of mice infected with HSV-IL-2 expressing IL-2rα and IL-2rβ but not expressing IL-2rγ. As expected, no demyelination was detected in the CNS of mice infected with control virus. In addition to be a receptor for IL-2, IL-2rγ is involved in generating ILC2s and NK cells (Wallrapp et al., 2018; Wong et al., 2012) and is a receptor for IL-4, IL-7, IL-9, IL-15, and IL-21 (Waldmann, 2006). Thus, the absence of demyelination in IL-2rγ−/− mice could be due to the absence of IL-2rγ binding to IL-2, the absence of ILC2s, the absence of NK cells, or the effect of IL-2rγ absence on IL-4, IL-7, IL-9, IL-15, and IL-21 functions. Thus, we looked at demyelination in brain, spinal cord, and ONs of ILC1−/−, ILC2−/−, and ILC3−/− mice infected with HSV-IL-2 or control virus. We did not find demyelination in the CNS of ILC2−/− mice infected ocularly with HSV-IL-2 virus, although demyelination was observed in ILC1−/−- and ILC3−/−-infected mice. These results showed that the absence of ILC2s in IL-2rγ−/− mice, not its binding to IL-2 or its effect on IL-4, IL-7, IL-9, IL-15, and IL-21 functions, contributed to HSV-IL-2-induced demyelination. To show that ILC2s specifically contribute to CNS demyelination in WT mice, we next performed adoptive transfer of BM-derived ILC2s to ILC2−/− mice and infected the reconstituted mice with HSV-IL-2 virus. As expected, we observed demyelination in the brains, spinal cords, and ONs of recipient ILC2−/− mice. Thus, CNS demyelination in HSV-IL-2-infected mice is due to the presence of ILC2s, not ILC1s or ILC3s. Quantification of the number, size, and shape of plaques in the CNS of IL-2rα−/−, IL2rβ−/−, ILC1−/−, ILC3−/−, or ILC2−/− mice that received ILC2 from WT mice were similar. Thus, this side by side comparison of CNS demyelination in different knockout mice did not show exacerbation of disease.
ILC2s include both a “natural” subset that is present during homeostasis and an “inflammatory” subset that is generated during an immune response (Sonnenberg and Hepworth, 2019). Although ILCs are found in limited numbers, they play important roles in protection and pathogenicity (Cording et al., 2016; Ebbo et al., 2017; Hams et al., 2014; Lee et al., 2015; Moral et al., 2020; Rauber et al., 2017; Salimi et al., 2013; Spits and Di Santo, 2011; Tait Wojno and Artis, 2016; Zook and Kee, 2016). Consistent with our study, ILC2s have been implicated in the development of allergy, asthma, dermatitis, and fibrosis (Cording et al., 2016; Ebbo et al., 2017; Hams et al., 2014; Salimi et al., 2013; Spits and Di Santo, 2011; Tait Wojno and Artis, 2016; Zook and Kee, 2016). ILC2s can induce airway hyper-reactivity independent of TH2 cells and adaptive immunity following lung infection with influenza A virus (Chang et al., 2011). ILC2s, which are the dominant ILCs in the lung, contribute to inflammation (Hurrell et al., 2019; Rigas et al., 2017) and are also the predominant ILC population in human and mouse brain (Cardoso et al., 2017; Klose et al., 2017; Wallrapp et al., 2017). Mucosal neurons regulate TH2 inflammation by releasing neuromeric U (NMU) that directly activates type 2 ILCs (Cardoso et al., 2017; Klose et al., 2017; Wallrapp et al., 2017). In this study we have shown that IL-2 expressed by HSV-IL-2 binds to ILC2s. Thus, similar to NMU, the binding of overexpressed IL-2 to the surface of ILC2s, combined with viral infection, may activate ILC2s leading to CNS demyelination. IL-2-deficient CD4+ T cells are known to more effectively control influenza A virus infection in the lung than do T cells that produce IL-2 (McKinstry et al., 2019). Thus, a combination of IL-2 expression and viral infection may induce pathogenesis in the CNS or lung of mice infected with HSV-IL-2 and influenza viruses, respectively.
Many factors have been implicated in protecting against or inducing MS and EAE with contradictory results depending on which model of CNS demyelination is used (Beck et al., 2003; Galli et al., 2019; Hafler, 2004; Haines et al., 1996; Hemmer et al., 2002; Hunter et al., 1997; Martin et al., 1992; Noseworthy et al., 2000; Zamvil and Steinman, 1990). Thus, we looked at the involvement of PD1, GM-CSF, IL-5, IL-6, IFNgr1, CCL5 (RANTES), CXCL10 (IP-10), 4-1BB/CD137, HIF1α, CSF2rb, Tim-3, and CTLA4 in demyelination or protection from demyelination in the brain of infected mice. Demyelination in both WT and ILC2−/− mice that received adoptive transfer of ILC2 from WT mice correlated with suppression of RANTES (also known as CCL5) and IP-10 (Interferon gamma-induced protein also known as C-X-C motif chemokine 10 (CXCL10). Our results suggest that in the presence of ILC2s, suppression of CCL5 and CXCL10 by a combination of IL-2 and viral infection correlated with demyelination. However, levels of both CCL5 and CXCL10 increased significantly in mice that were infected with parental virus or in ILC2−/− mice that were infected with HSV-IL-2. Previously we reported no expression of CCL5 or CXCL10 in ILC1 or ILC3 cells with and without infection with WT HSV-1, whereas both CCL5 and CXCL10 were significantly upregulated in HSV-1-infected ILC2 (Hirose et al., 2019). Similar to this study and our previous report, CXCL10 is elevated following infection with hepatitis C virus and HIV (Falconer et al., 2010; Lagging et al., 2006). In contrast to our study, CXCL10+ cells were recently shown to be pathogenic in the CNS of EAE mice (Giladi et al., 2020). CCL5 plays a primary role in the inflammatory immune response and has been implicated in EAE and MS (Gonzalez-Amaro and Sanchez-Madrid, 2002; Jee et al., 2002). Overall, the role of CCL5 and CXCL10 in inflammatory autoimmunity, particularly in neuroinflammation, is controversial (Karin, 2020; Lalor and Segal, 2013; Mills Ko et al., 2014; Muller et al., 2010). Both CCL5 and CXCL10 are chemotactic for T cells and play an active role in recruiting leukocytes into inflammatory sites. We previously showed that CD4+ and CD8+ T cells are both involved in HSV-IL-2-induced CNS demyelination (Zandian et al., 2011a). In this study we showed that the presence of ILC2s is required for CNS demyelination. Similarly, previous studies showed that ILC2s in different tissues and in response to the local environment, selectively express distinct cytokine patterns for cell activation (Ricardo-Gonzalez et al., 2018). ILC2s have been shown to express MHCII, CD80, CD86, and OX40L and also to act as APCs for antigen presentation to T cells (Halim et al., 2018; Maazi et al., 2015; Oliphant et al., 2014). Thus, specific ILC2s activation may contribute to protection or disease as previously reported (Cording et al., 2016; Ebbo et al., 2017; Hams et al., 2014; Lee et al., 2015; Rauber et al., 2017; Salimi et al., 2013; Spits and Di Santo, 2011; Tait Wojno and Artis, 2016; Zook and Kee, 2016). Our current study suggests communication between ILC2s and T cells via IL-2 produced by HSV-IL-2 and virus infection function as a critical enhancer of T cell autoreactivity. However, the absence of ILC2s can block the effects of HSV-IL-2 on CNS pathology due to elevated expression of CCL5 and CXCL10, which are defensive molecules produced by neurons to recruit protective T cells. In contrast, suppression of CCL5 and CXCL10 in the presence of ILC2 by HSV-IL-2 may alter the T cell phenotype, and these pathogenic T cells may cause CNS demyelination.
Previously we have shown that FoxP3+ T cells contribute to CNS demyelination and depletion of FoxP3 blocks CNS demyelination by HSV-IL-2 (Zandian et al., 2011a). Our previous studies also have shown that HSV-IL-2-induced CNS demyelination was associated with T cells having a CD62LhiCD45RBloFoxP3hi, whereas the absence of demyelination was associated with CD62LloCD45RBhiFoxP3lo (Osorio et al., 2005; Zandian et al., 2011a).
In summary, the results presented here are in line with our previously studies (Dumitrascu et al., 2014; Mott et al., 2011, 2013; Osorio et al., 2005; Zandian et al., 2009, 2011a) and suggest that a combination of HSV-1 infection and overexpression of IL-2 is responsible for dysregulation of ILC2 and reduced expression of chemotactic cytokines CCL5 and CXCL10, leading to enhanced recruitment of pathogenic T cells (i.e., CD62LhiCD45RBloFoxP3hi) and thus CNS demyelination.
Limitations of the Study
As our studies were done using a mouse model, it is currently not known if these results are applicable to humans. Future studies using ILC2s isolated from MS patients or cadavers should be done to validate our findings.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Homayon Ghiasi (ghiasih@cshs.org).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate datasets or analyze codes.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This study was supported by Public Health Service NIH grants R01EY029677, R01EY013615 and RO1EY026944.
Author Contributions
SH and HG conceived the studies. SH, PSJ, and UJ performed experiments and generated primary data, including developing methodology, validation, and data curation. SH and HG performed formal analysis and visualization. SH, SW, and JU assisted in animal colony maintenance and performing some mouse experiments. SH performed and analyzed transcriptional profiling data. SH, KT, OA, and HG contributed to writing the manuscript. All authors contributed to reviewing and editing the final manuscript. HG was responsible for project supervision, administration, and funding acquisition.
Declaration of Interests
The authors declare no competing interests.
Published: October 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101549.
Supplemental Information
References
- Abt M.C., Buffie C.G., Susac B., Becattini S., Carter R.A., Leiner I., Keith J.W., Artis D., Osborne L.C., Pamer E.G. TLR-7 activation enhances IL-22-mediated colonization resistance against vancomycin-resistant enterococcus. Sci. Transl. Med. 2016;8:327ra325. doi: 10.1126/scitranslmed.aad6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt M.C., McKenney P.T., Pamer E.G. Clostridium difficile colitis: pathogenesis and host defence. Nat. Rev. Microbiol. 2016;14:609–620. doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi K., Kumamoto T., Araki S. Interleukin-2 receptor levels indicating relapse in multiple sclerosis. Lancet. 1989;1:559–560. doi: 10.1016/s0140-6736(89)90103-7. [DOI] [PubMed] [Google Scholar]
- Bansil S., Troiano R., Cook S.D., Rohowsky-Kochan C. Serum soluble interleukin-2 receptor levels in chronic progressive, stable and steroid-treated multiple sclerosis. Acta Neurol. Scand. 1991;84:282–285. doi: 10.1111/j.1600-0404.1991.tb04955.x. [DOI] [PubMed] [Google Scholar]
- Beck R.W., Trobe J.D., Moke P.S., Gal R.L., Xing D., Bhatti M.T., Brodsky M.C., Buckley E.G., Chrousos G.A., Corbett J. High- and low-risk profiles for the development of multiple sclerosis within 10 years after optic neuritis: experience of the optic neuritis treatment trial. Arch. Ophthalmol. 2003;121:944–949. doi: 10.1001/archopht.121.7.944. [DOI] [PubMed] [Google Scholar]
- Boman J., Roblin P.M., Sundstrom P., Sandstrom M., Hammerschlag M.R. Failure to detect Chlamydia pneumoniae in the central nervous system of patients with MS. Neurology. 2000;54:265. doi: 10.1212/wnl.54.1.265. [DOI] [PubMed] [Google Scholar]
- Bureau J.F., Drescher K.M., Pease L.R., Vikoren T., Delcroix M., Zoecklein L., Brahic M., Rodriguez M. Chromosome 14 contains determinants that regulate susceptibility to Theiler's virus-induced demyelination in the mouse. Genetics. 1998;148:1941–1949. doi: 10.1093/genetics/148.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Shores E.W., Hu-Li J., Anver M.R., Kelsall B.L., Russell S.M., Drago J., Noguchi M., Grinberg A., Bloom E.T. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Cardoso V., Chesne J., Ribeiro H., Garcia-Cassani B., Carvalho T., Bouchery T., Shah K., Barbosa-Morais N.L., Harris N., Veiga-Fernandes H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549:277–281. doi: 10.1038/nature23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challoner P.B., Smith K.T., Parker J.D., MacLeod D.L., Coulter S.N., Rose T.M., Schultz E.R., Bennett J.L., Garber R.L., Chang M. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl. Acad. Sci. U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.J., Kim H.Y., Albacker L.A., Baumgarth N., McKenzie A.N., Smith D.E., Dekruyff R.H., Umetsu D.T. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cording S., Medvedovic J., Aychek T., Eberl G. Innate lymphoid cells in defense, immunopathology and immunotherapy. Nat. Immunol. 2016;17:755–757. doi: 10.1038/ni.3448. [DOI] [PubMed] [Google Scholar]
- Cua D.J., Groux H., Hinton D.R., Stohlman S.A., Coffman R.L. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daibata M., Komatsu T., Taguchi H. Human herpesviruses in primary ocular lymphoma. Leuk. Lymphoma. 2000;37:361–365. doi: 10.3109/10428190009089436. [DOI] [PubMed] [Google Scholar]
- Dumitrascu O.M., Mott K.R., Ghiasi H. A comparative study of experimental mouse models of central nervous system demyelination. Gene Ther. 2014;21:599–608. doi: 10.1038/gt.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbo M., Crinier A., Vely F., Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat. Rev. Immunol. 2017;17:665–678. doi: 10.1038/nri.2017.86. [DOI] [PubMed] [Google Scholar]
- Falconer K., Askarieh G., Weis N., Hellstrand K., Alaeus A., Lagging M. IP-10 predicts the first phase decline of HCV RNA and overall viral response to therapy in patients co-infected with chronic hepatitis C virus infection and HIV. Scand. J. Infect. Dis. 2010;42:896–901. doi: 10.3109/00365548.2010.498019. [DOI] [PubMed] [Google Scholar]
- Ferrante P., Mancuso R., Pagani E., Guerini F.R., Calvo M.G., Saresella M., Speciale L., Caputo D. Molecular evidences for a role of HSV-1 in multiple sclerosis clinical acute attack. J. Neurovirol. 2000;6(Suppl 2):S109–S114. [PubMed] [Google Scholar]
- Friedman J.E., Lyons M.J., Cu G., Ablashl D.V., Whitman J.E., Edgar M., Koskiniemi M., Vaheri A., Zabriskie J.B. The association of the human herpesvirus-6 and MS. Mult. Scler. 1999;5:355–362. doi: 10.1177/135245859900500509. [DOI] [PubMed] [Google Scholar]
- Galli E., Hartmann F.J., Schreiner B., Ingelfinger F., Arvaniti E., Diebold M., Mrdjen D., van der Meer F., Krieg C., Nimer F.A. GM-CSF and CXCR4 define a T helper cell signature in multiple sclerosis. Nat. Med. 2019;25:1290–1300. doi: 10.1038/s41591-019-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo P., Piccinno M., Pagni S., Tavolato B. Interleukin-2 levels in serum and cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 1988;24:795–797. doi: 10.1002/ana.410240618. [DOI] [PubMed] [Google Scholar]
- Gallo P., Piccinno M.G., Pagni S., Argentiero V., Giometto B., Bozza F., Tavolato B. Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and gamma-IFN levels in serum and cerebrospinal fluid. J. Neurol. Sci. 1989;92:9–15. doi: 10.1016/0022-510x(89)90171-8. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Osorio Y., Hedvat Y., Perng G.C., Nesburn A.B., Wechsler S.L. Infection of BALB/c mice with a herpes simplex virus type 1 recombinant virus expressing IFN-g driven by the LAT promoter. Virology. 2002;302:144–154. doi: 10.1006/viro.2002.1609. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Osorio Y., Perng G.C., Nesburn A.B., Wechsler S.L. Recombinant herpes simplex virus type 1 expressing murine interleukin-4 is less virulent than wild-type virus in mice. J. Virol. 2001;75:9029–9036. doi: 10.1128/JVI.75.19.9029-9036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi H., Osorio Y., Perng G.C., Nesburn A.B., Wechsler S.L. Overexpression of interleukin-2 by a recombinant herpes simplex virus type 1 attenuates pathogenicity and enhances antiviral immunity. J. Virol. 2002;76:9069–9078. doi: 10.1128/JVI.76.18.9069-9078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi A., Wagner L.K., Li H., Dorr D., Medaglia C., Paul F., Shemer A., Jung S., Yona S., Mack M. Cxcl10(+) monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat. Immunol. 2020;21:525–534. doi: 10.1038/s41590-020-0661-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Amaro R., Sanchez-Madrid F. [Intercellular adhesion molecules and chemotaxic factors in the pathogenesis of multiple sclerosis] Rev. Neurol. 2002;35:985–993. [PubMed] [Google Scholar]
- Greenberg S.J., Marcon L., Hurwitz B.J., Waldmann T.A., Nelson D.L. Elevated levels of soluble interleukin-2 receptors in multiple sclerosis. N. Engl. J. Med. 1988;319:1019–1020. doi: 10.1056/NEJM198810133191517. [DOI] [PubMed] [Google Scholar]
- Hafler D.A. Multiple sclerosis. J. Clin. Invest. 2004;113:788–794. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J.L., Ter-Minassian M., Bazyk A., Gusella J.F., Kim D.J., Terwedow H., Pericak-Vance M.A., Rimmler J.B., Haynes C.S., Roses A.D. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group. Nat. Genet. 1996;13:469–471. doi: 10.1038/ng0896-469. [DOI] [PubMed] [Google Scholar]
- Halim T.Y.F., Rana B.M.J., Walker J.A., Kerscher B., Knolle M.D., Jolin H.E., Serrao E.M., Haim-Vilmovsky L., Teichmann S.A., Rodewald H.R. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity. 2018;48:1195–1207 e1196. doi: 10.1016/j.immuni.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams E., Armstrong M.E., Barlow J.L., Saunders S.P., Schwartz C., Cooke G., Fahy R.J., Crotty T.B., Hirani N., Flynn R.J. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl. Acad. Sci. U S A. 2014;111:367–372. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H.P., Hughes R.A., Taylor W.A., Heininger K., Reiners K., Toyka K.V. T cell activation in Guillain-Barre syndrome and in MS: elevated serum levels of soluble IL-2 receptors. Neurology. 1990;40:215–218. doi: 10.1212/wnl.40.2.215. [DOI] [PubMed] [Google Scholar]
- Hemmer B., Cepok S., Nessler S., Sommer N. Pathogenesis of multiple sclerosis: an update on immunology. Curr. Opin. Neurol. 2002;15:227–231. doi: 10.1097/00019052-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Hirose S., Wang S., Tormanen K., Wang Y., Tang J., Akbari O., Ghiasi H. Roles of type 1, 2, and 3 innate lymphoid cells in herpes simplex virus 1 infection in vitro and in vivo. J. Virol. 2019;93 doi: 10.1128/JVI.00523-19. e00523–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S.F., Weinshenker B.G., Carter J.L., Noseworthy J.H. Rational clinical immunotherapy for multiple sclerosis. Mayo Clin. Proc. 1997;72:765–780. doi: 10.1016/S0025-6196(11)63598-2. [DOI] [PubMed] [Google Scholar]
- Hurrell B.P., Galle-Treger L., Jahani P.S., Howard E., Helou D.G., Banie H., Soroosh P., Akbari O. TNFR2 signaling enhances ILC2 survival, function, and induction of airway hyperreactivity. Cell Rep. 2019;29:4509–4524 e4505. doi: 10.1016/j.celrep.2019.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee Y., Yoon W.K., Okura Y., Tanuma N., Matsumoto Y. Upregulation of monocyte chemotactic protein-1 and CC chemokine receptor 2 in the central nervous system is closely associated with relapse of autoimmune encephalomyelitis in Lewis rats. J. Neuroimmunol. 2002;128:49–57. doi: 10.1016/s0165-5728(02)00147-9. [DOI] [PubMed] [Google Scholar]
- Karin N. CXCR3 ligands in cancer and autoimmunity, chemoattraction of effector T cells, and beyond. Front. Immunol. 2020;11:976. doi: 10.3389/fimmu.2020.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittur S.D., Kittur D.S., Soncrant T.T., Rapoport S.I., Tourtellotte W.W., Nagel J.E., Adler W.H. Soluble interleukin-2 receptors in cerebrospinal fluid from individuals with various neurological disorders. Ann. Neurol. 1990;28:168–173. doi: 10.1002/ana.410280209. [DOI] [PubMed] [Google Scholar]
- Klose C.S.N., Mahlakoiv T., Moeller J.B., Rankin L.C., Flamar A.L., Kabata H., Monticelli L.A., Moriyama S., Putzel G.G., Rakhilin N. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549:282–286. doi: 10.1038/nature23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K.K., Brewer J.H., Henry J.M., Harrington D.J., Carrigan D.R. Human herpesvirus 6 and multiple sclerosis: systemic active infections in patients with early disease. Clin. Infect. Dis. 2000;31:894–903. doi: 10.1086/318141. [DOI] [PubMed] [Google Scholar]
- Lagging M., Romero A.I., Westin J., Norkrans G., Dhillon A.P., Pawlotsky J.M., Zeuzem S., von Wagner M., Negro F., Schalm S.W. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006;44:1617–1625. doi: 10.1002/hep.21407. [DOI] [PubMed] [Google Scholar]
- Lalor S.J., Segal B.M. Th1-mediated experimental autoimmune encephalomyelitis is CXCR3 independent. Eur. J. Immunol. 2013;43:2866–2874. doi: 10.1002/eji.201343499. [DOI] [PubMed] [Google Scholar]
- Lee M.W., Odegaard J.I., Mukundan L., Qiu Y., Molofsky A.B., Nussbaum J.C., Yun K., Locksley R.M., Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.Z., Fredrikson S., Xiao B.G., Link H. Interleukin-2 secreting cells in multiple sclerosis and controls. J. Neurol. Sci. 1993;120:99–106. doi: 10.1016/0022-510x(93)90032-t. [DOI] [PubMed] [Google Scholar]
- Maazi H., Patel N., Sankaranarayanan I., Suzuki Y., Rigas D., Soroosh P., Freeman G.J., Sharpe A.H., Akbari O. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42:538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Enbom M., Soderstrom M., Fredrikson S., Dahl H., Lycke J., Bergstrom T., Linde A. Absence of seven human herpesviruses, including HHV-6, by polymerase chain reaction in CSF and blood from patients with multiple sclerosis and optic neuritis. Acta Neurol. Scand. 1997;95:280–283. doi: 10.1111/j.1600-0404.1997.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Martin R., McFarland H.F., McFarlin D.E. Immunological aspects of demyelinating diseases. Annu. Rev. Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- McCombe P.A., Nickson I., Pender M.P. Cytokine expression by inflammatory cells obtained from the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis induced by inoculation with myelin basic protein and adjuvants. J. Neuroimmunol. 1998;88:30–38. doi: 10.1016/s0165-5728(98)00068-x. [DOI] [PubMed] [Google Scholar]
- McKenzie A.N.J., Spits H., Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–374. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- McKinstry K.K., Alam F., Flores-Malavet V., Nagy M.Z., Sell S., Cooper A.M., Swain S.L., Strutt T.M. Memory CD4 T cell-derived IL-2 synergizes with viral infection to exacerbate lung inflammation. PLoS Pathog. 2019;15:e1007989. doi: 10.1371/journal.ppat.1007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills Ko E., Ma J.H., Guo F., Miers L., Lee E., Bannerman P., Burns T., Ko D., Sohn J., Soulika A.M., Pleasure D. Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J. Neuroinflammation. 2014;11:105. doi: 10.1186/1742-2094-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu. Rev. Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Mirandola P., Stefan A., Brambilla E., Campadelli-Fiume G., Grimaldi L.M. Absence of human herpesvirus 6 and 7 from spinal fluid and serum of multiple sclerosis patients. Neurology. 1999;53:1367–1368. doi: 10.1212/wnl.53.6.1367-a. [DOI] [PubMed] [Google Scholar]
- Moral J.A., Leung J., Rojas L.A., Ruan J., Zhao J., Sethna Z., Ramnarain A., Gasmi B., Gururajan M., Redmond D. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature. 2020;579:130–135. doi: 10.1038/s41586-020-2015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K.R., Gate D., Zandian M., Allen S.J., Rajasagi N.K., Van Rooijen N., Chen S.C., Arditi M., Rouse B.T., Flavell R.A. Macrophage IL-12p70 signaling prevents HSV-1-induced CNS autoimmunity triggered by autoaggressive CD4+ Tregs. Invest. Ophthalmol. Vis. Sci. 2011;52:2321–2333. doi: 10.1167/iovs.10-6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K.R., Maazi H., Allen S.J., Zandian M., Matundan H., Ghiasi Y.N., Sharifi B.G., Underhill D., Akbari O., Ghiasi H. Batf3 deficiency is not critical for the generation of CD8alpha(+) dendritic cells. Immunobiology. 2015;220:518–524. doi: 10.1016/j.imbio.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K.R., Osorio Y., Brown D.J., Morishige N., Wahlert A., Jester J.V., Ghiasi H. The corneas of naive mice contain both CD4+ and CD8+ T cells. Mol. Vis. 2007;13:1802–1812. [PubMed] [Google Scholar]
- Mott K.R., Perng G.C., Osorio Y., Kousoulas K.G., Ghiasi H. A recombinant herpes simplex virus type 1 expressing two additional copies of gK is more pathogenic than wild-type virus in two different strains of mice. J. Virol. 2007;81:12962–12972. doi: 10.1128/JVI.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K.R., Zandian M., Allen S.J., Ghiasi H. Role of IL-2 and HSV-1 in CNS demyelination in mice. J. Virol. 2013;87:12102–12109. doi: 10.1128/JVI.02241-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M., Carter S., Hofer M.J., Campbell I.L. Review: the chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity--a tale of conflict and conundrum. Neuropathol. Appl. Neurobiol. 2010;36:368–387. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- Nicoll J.A., Kinrade E., Love S. PCR-mediated search for herpes simplex virus DNA in sections of brain from patients with multiple sclerosis and other neurological disorders. J. Neurol. Sci. 1992;113:144–151. doi: 10.1016/0022-510x(92)90242-d. [DOI] [PubMed] [Google Scholar]
- Noseworthy J.H., Lucchinetti C., Rodriguez M., Weinshenker B.G. Multiple sclerosis. N. Engl. J. Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Oliphant C.J., Hwang Y.Y., Walker J.A., Salimi M., Wong S.H., Brewer J.M., Englezakis A., Barlow J.L., Hams E., Scanlon S.T. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio Y., Ghiasi H. Comparison of adjuvant efficacy of herpes simplex virus type 1 recombinant viruses expressing TH1 and TH2 cytokine genes. J. Virol. 2003;77:5774–5783. doi: 10.1128/JVI.77.10.5774-5783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio Y., La Point S.F., Nusinowitz S., Hofman F.M., Ghiasi H. CD8+-dependent CNS demyelination following ocular infection of mice with a recombinant HSV-1 expressing murine IL-2. Exp. Neurol. 2005;193:1–18. doi: 10.1016/j.expneurol.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Osorio Y., Sharifi B.G., Perng G.C., Ghiasi N.S., Ghiasi H. The role of TH1 and TH2 cytokines in HSV-1-induced corneal scarring. Ocul. Immunol. Inflamm. 2003;10:105–116. doi: 10.1076/ocii.10.2.105.13982. [DOI] [PubMed] [Google Scholar]
- Petitto J.M., Streit W.J., Huang Z., Butfiloski E., Schiffenbauer J. Interleukin-2 gene deletion produces a robust reduction in susceptibility to experimental autoimmune encephalomyelitis in C57BL/6 mice. Neurosci. Lett. 2000;285:66–70. doi: 10.1016/s0304-3940(00)00996-4. [DOI] [PubMed] [Google Scholar]
- Rafei-Shamsabadi D.A., van de Poel S., Dorn B., Kunz S., Martin S.F., Klose C.S.N., Arnold S.J., Tanriver Y., Ebert K., Diefenbach A. Lack of type 2 innate lymphoid cells promotes a type I-driven enhanced immune response in Contact hypersensitivity. J. Invest. Dermatol. 2018;138:1962–1972. doi: 10.1016/j.jid.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauber S., Luber M., Weber S., Maul L., Soare A., Wohlfahrt T., Lin N.Y., Dietel K., Bozec A., Herrmann M. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat. Med. 2017;23:938–944. doi: 10.1038/nm.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Gonzalez R.R., Van Dyken S.J., Schneider C., Lee J., Nussbaum J.C., Liang H.E., Vaka D., Eckalbar W.L., Molofsky A.B., Erle D.J., Locksley R.M. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 2018;19:1093–1099. doi: 10.1038/s41590-018-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas D., Lewis G., Aron J.L., Wang B., Banie H., Sankaranarayanan I., Galle-Treger L., Maazi H., Lo R., Freeman G.J. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J. Allergy Clin. Immunol. 2017;139:1468–1477 e1462. doi: 10.1016/j.jaci.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B., Kyle R., Tay S.S., Mitchell A.J., Bolton H.A., Guy T.V., Tan S.Y., Forbes-Blom E., Tong P.L., Koller Y. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J. Allergy Clin. Immunol. 2015;136:1653–1663 e1657. doi: 10.1016/j.jaci.2015.03.043. [DOI] [PubMed] [Google Scholar]
- Salimi M., Barlow J.L., Saunders S.P., Xue L., Gutowska-Owsiak D., Wang X., Huang L.C., Johnson D., Scanlon S.T., McKenzie A.N. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillet C., Belz G.T., Huntington N.D. Development, homeostasis, and heterogeneity of NK cells and ILC1. Curr. Top. Microbiol. Immunol. 2016;395:37–61. doi: 10.1007/82_2015_474. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Nowak Z., Tchorzewski H. Interleukin-1 and interleukin-2 production by peripheral blood mononuclear cells in multiple sclerosis patients. J. Neurol. Sci. 1988;85:67–76. doi: 10.1016/0022-510x(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Nowak Z., Tchorzewski H. Multiple sclerosis: effect of myelin basic protein on interleukin 1, interleukin 2 production and interleukin 2 receptor expression in vitro. Clin. Exp. Immunol. 1988;72:428–433. [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.F., Hepworth M.R. Functional interactions between innate lymphoid cells and adaptive immunity. Nat. Rev. Immunol. 2019;19:599–613. doi: 10.1038/s41577-019-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M., Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Spits H., Di Santo J.P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- Tait Wojno E.D., Artis D. Emerging concepts and future challenges in innate lymphoid cell biology. J. Exp. Med. 2016;213:2229–2248. doi: 10.1084/jem.20160525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taus C., Pucci E., Cartechini E., Fie A., Giuliani G., Clementi M., Menzo S. Absence of HHV-6 and HHV-7 in cerebrospinal fluid in relapsing- remitting multiple sclerosis. Acta Neurol. Scand. 2000;101:224–228. doi: 10.1034/j.1600-0404.2000.101004224.x. [DOI] [PubMed] [Google Scholar]
- Traugott U. Multiple sclerosis: relevance of class I and class II MHC-expressing cells to lesion development. J. Neuroimmunol. 1987;16:283–302. doi: 10.1016/0165-5728(87)90082-8. [DOI] [PubMed] [Google Scholar]
- Trotter J.L., Clifford D.B., McInnis J.E., Griffeth R.C., Bruns K.A., Perlmutter M.S., Anderson C.B., Collins K.G., Banks G., Hicks B.C. Correlation of immunological studies and disease progression in chronic progressive multiple sclerosis. Ann. Neurol. 1989;25:172–178. doi: 10.1002/ana.410250211. [DOI] [PubMed] [Google Scholar]
- Waldmann T.A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- Wallrapp A., Riesenfeld S.J., Burkett P.R., Abdulnour R.E., Nyman J., Dionne D., Hofree M., Cuoco M.S., Rodman C., Farouq D. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549:351–356. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrapp A., Riesenfeld S.J., Burkett P.R., Kuchroo V.K. Type 2 innate lymphoid cells in the induction and resolution of tissue inflammation. Immunol. Rev. 2018;286:53–73. doi: 10.1111/imr.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.H., Walker J.A., Jolin H.E., Drynan L.F., Hams E., Camelo A., Barlow J.L., Neill D.R., Panova V., Koch U. Transcription factor RORalpha is critical for nuocyte development. Nat. Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Lindsberg P.J., Hukkanen V., Seljelid R., Gahmberg C.G., Meri S. Differential expression of cytokines (IL-2, IFN-gamma, IL-10) and adhesion molecules (VCAM-1, LFA-1, CD44) between spleen and lymph nodes associates with remission in chronic relapsing experimental autoimmune encephalomyelitis. Scand. J. Immunol. 2002;56:286–293. doi: 10.1046/j.1365-3083.2002.01132.x. [DOI] [PubMed] [Google Scholar]
- Yokota Y., Mansouri A., Mori S., Sugawara S., Adachi S., Nishikawa S., Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- Zamvil S.S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zandian M., Belisle R., Mott K.R., Nusinowitz S., Hofman F.M., Ghiasi H. Optic neuritis in different strains of mice by a recombinant HSV-1 expressing murine interleukin-2. Invest. Ophthalmol. Vis. Sci. 2009;50:3275–3282. doi: 10.1167/iovs.08-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandian M., Mott K.R., Allen S.J., Chen S., Arditi M., Ghiasi H. IL-2 suppression of IL-12p70 by a a recombinant HSV-1 expressing IL-2 induces T cells auto-reactivity and CNS demyelination. PLoS One. 2011;6:e16820. doi: 10.1371/journal.pone.0016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandian M., Mott K.R., Allen S.J., Dumitrascu O., Kuo J.Z., Ghiasi H. Use of cytokine immunotherapy to block CNS demyelination induced by a recombinant HSV-1 expressing murine interleukin-2. Gene Ther. 2011;18:734–742. doi: 10.1038/gt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook E.C., Kee B.L. Development of innate lymphoid cells. Nat. Immunol. 2016;17:775–782. doi: 10.1038/ni.3481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets or analyze codes.