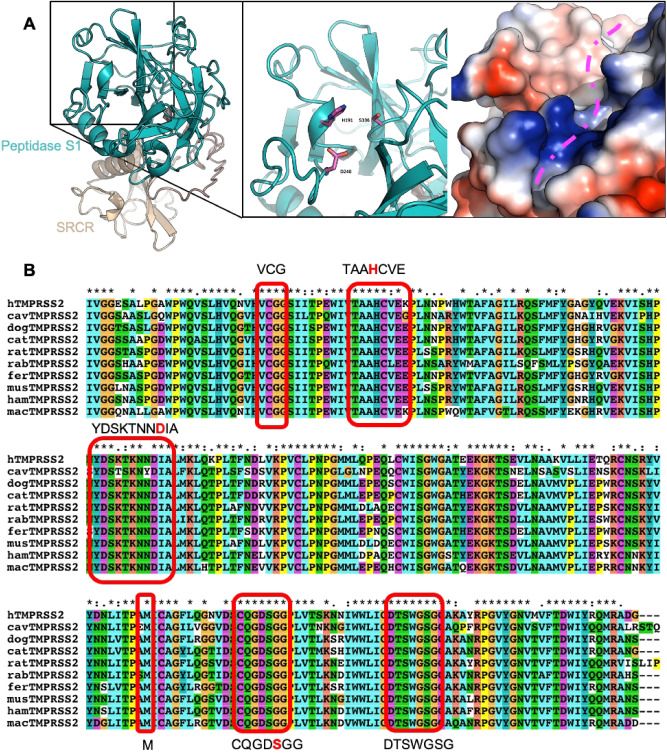
Figure 3.
The TMPRSS2 active site is highly conserved among species. (A) Cartoon representation of hTMPRSS2, with the SRCR domain in beige and the Peptidase S1 domain in teal. In the close-up panels the catalytic triad (H296, D345 and S441) is shown in violet sticks and molecular surface colored by electrostatic potential (from − 44 kT/e (red) to 44 kT/e (blue)). A pink dotted line has been placed in the Peptidase S1 active pocket. (B) Multiple sequences alignment of hTMPRSS2, cavTMPRSS2, dogTMPRSS2, catTMPRSS2, ratTMPRSS2, rabTMPRSS2, ferTMPRSS2, musTMPRSS2. Peptidase S1 active pocket residues have been highlighted in red with the relative consensus sequence. Catalytic triad residues are shown in red in the consensus sequence.