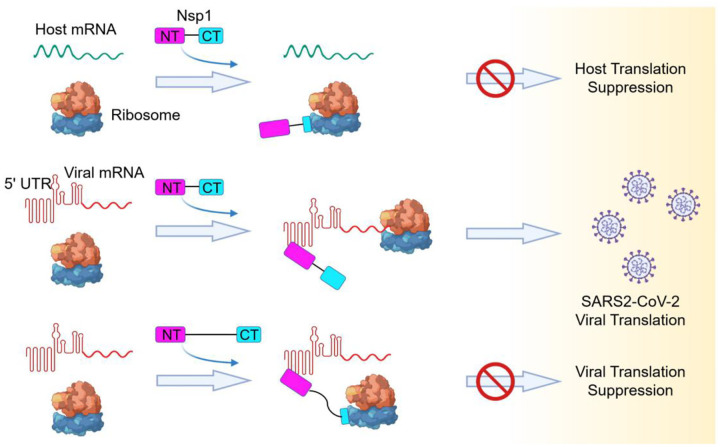
Figure 5. Model of a Bipartite Mechanism for Nsp1-Mediated Translation Inhibition and Evasion by SARS-CoV-2 5’ UTR.
A schematic model depicting the bipartite roles of SARS-CoV-2 Nsp1 during infection. First, Nsp1 blocks host mRNA from binding to the 40S ribosomal subunit due to physical occlusion by the bound Nsp1-CT. Second, Nsp1 supports viral mRNA translation by interacting with SARS-CoV-2 5’ UTR using Nsp1-NT, which results in dissociation of the Nsp1-CT-40S complex to overcome inhibition. This mechanism of evasion of Nsp1-mediated translation inhibition is illustrated by the failure of linker-lengthened Nsp1 to support viral mRNA translation. With the longer linker, the Nsp1-NT-5’ UTR complex can co-exist with the Nsp1-CT-40S complex.