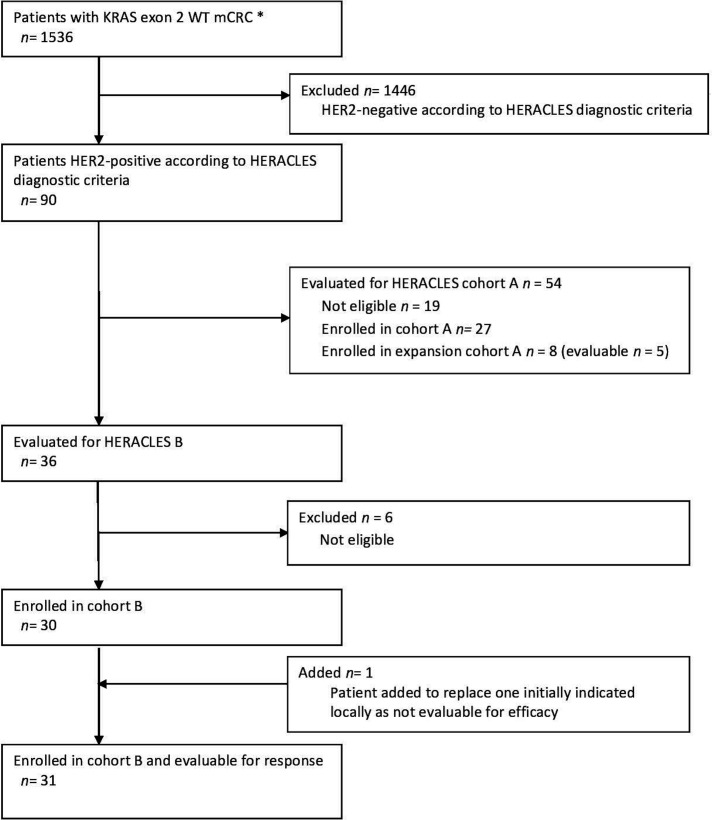
Figure 2.
Consolidated Standards of Reporting Trials diagram of HERACLES-B trial. Between August 2012 and March 2018, 1536 patients with KRAS exon 2 and BRAF wild type (WT) (from 15 March 2016 exons 2, 3, 4 KRAS and NRAS and BRAF WT) were screened by immunohistochemistry (IHC) and in situ hybridisation (ISH) as per HERACLES criteria. Ninety patients (5.9%) had a HER2+ tumour. HERACLES cohort A comprised overall 35 patients (27 original cohort plus 8 expansion cohort), of whom 32 were evaluable for response.8 Enrolment in cohort B (HERACLES B trial) started in August 2016, data were collected until March 2019 and final analysis and centralised radiological revision were completed by 30 July 2019. One patient signed consent but developed rapid disease progression and was locally indicated as not evaluable for efficacy. The patients was duly substituted as per protocol. However, the centralised revision of the treatment history subsequently revealed instead that patient was fully evaluable for both safety and efficacy as for protocol since two full cycles of treatment were delivered. For this reason, all analyses have been performed on 31 rather than 30 patients as originally intended. *From 15 March 2016 RAS WT (KRAS, NRAS exon 2, 3, 4).