Abstract
BACKGROUND:
Pelvic lymph node metastasis carries the highest impact on decreased survival among surgical–pathological risk factors for early-stage cervical cancer. Although concurrent administration of chemotherapy during postoperative radiotherapy is the current standard treatment for surgically treated high-risk early-stage cervical cancer, its effectiveness specific to node-positive disease has not been completely studied.
OBJECTIVE:
To examine the association between the use of concurrent chemotherapy and survival in women with early-stage cervical cancer and nodal metastasis receiving adjuvant radiotherapy.
MATERIALS AND METHODS:
This is a population-based cohort study using the Surveillance, Epidemiology, and End Results Program from 1988 to 2016. Women with stage T1–2 cervical cancer with pelvic lymph node metastasis who underwent hysterectomy and received postoperative radiotherapy were examined. Trends, characteristics, and overall survival were compared between women who received postoperative radiotherapy alone (n = 729) or in combination with concurrent chemo-radiotherapy (n = 1809). Propensity score–based inverse probability of treatment weighting was used to account for the effect of measured covariates on treatment selection.
RESULTS:
Among 2538 women, there was a marked increase in the use of concurrent chemotherapy from 1997 to 2000 (20.7% to 78.5%, P = .052), followed by a more gradual rise through 2016 (88.3%, P < .001). In a multivariable model, women with non–squamous cell carcinomas and those diagnosed more recently were more likely to receive concurrent chemo-radiotherapy, whereas older women were less likely to receive concurrent chemo-radiotherapy (all, P < .05). At the population level, the 5-year overall survival rates remained unchanged (annual percent change for 1997–2012: −0.1; 95% confidence interval, −1.2 to 1.0; P = .776). In a propensity score weighted cohort, women who received concurrent chemo-radiotherapy had a 5-year overall survival rate similar to women treated with radiotherapy alone (73.1% vs 73.6%; hazard ratio, 1.004; 95% confidence interval, 0.887–1.136; P = .955). Significant differences were also not seen in older women, nonsquamous types, stage T2 disease, and multiple node metastases (all, P > .05).
CONCLUSION:
Despite the marked increase in the use of concurrent chemo-radiotherapy for women with early-stage cervical cancer and nodal metastases, there was no association between use of concurrent chemotherapy during postoperative radiotherapy and improved survival.
Keywords: adjuvant therapy, concurrent chemo-radiotherapy, cervical cancer, high risk, lymph node metastasis, survival
Cervical cancer is the third most common gynecologic malignancy in the United States, with an estimated 13,170 new diagnoses in 2019.1 Treatment of women with cervical cancer is generally based on the extent of disease. Radical hysterectomy is the surgical treatment of choice for women with stage IA2–IB1 tumors.2 Histopathological information obtained from the surgical specimen is useful to identify patients at increased risk for disease relapse and mortality. Historically, surgical–pathological risk factors have been categorized into 3 groups (low-, intermediate-, and high-risk),3-5 and tailored postoperative therapy is recommended based on the risk category.2
In addition to parametrial involvement and positive surgical margins, pelvic lymph node metastasis represents 1 of the 3 recognized high-risk surgical–pathological factors in cervical cancer, and is found in up to one-fourth of stage T1–2 cases.2,3,6,7 This has the highest impact on adverse disease outcome among the 3 high-risk factors, and in early-stage cervical cancer it confers a substantial risk of distant metastasis (22.3–24.2%).8,9
Postoperative radiotherapy has long been used for women with high-risk features after hysterectomy.2,10,11 Concomitant administration of chemotherapy during postoperative radiotherapy is the current standard of care, as it enhances the treatment effectiveness. This is based on the results of a randomized controlled trial for high-risk early-stage cervical cancer that demonstrated a 50% decrease in all-cause mortality in women who received concurrent chemo-radiotherapy (CCRT) compared to those treated with radiotherapy alone.3 The results of this trial and others prompted the National Cancer Institute (NCI) to issue a special announcement in 1999 recommending cisplatin-based chemotherapy with radiotherapy for women with cervical cancer.12,13
Despite the superiority of CCRT over radiotherapy alone in a randomized controlled trial, recent observational data have suggested a more modest benefit for combined-modality therapy in the setting of node-positive early-stage disease. A national cohort study from Japan demonstrated that recurrence rates, distant failure, and mortality were similar between women with early-stage cervical cancer and nodal metastases treated with CCRT compared to radiotherapy alone.9 These findings suggest that CCRT for node-positive high-risk early-stage cervical cancer may not be adequate for disease control. Data describing population-based trends and outcomes of CCRT use for node-positive early-stage cervical cancer patients in the United States has been missing.
The objective of our study was to examine the effect of concomitant chemotherapy on survival of women who received postoperative radiotherapy for high-risk early-stage cervical cancer with lymph node metastasis.
Materials and Methods
Data source
This is a retrospective observational study examining the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program data between 1988 and 2016.14 The SEER Program is the largest population-based tumor registry in the United States and has been in operation since 1973. The program covers nearly 34.6% of the US population in the latest version, and their data are publicly available and deidentified.
Patient identification, data entry, and quality control for this database are managed by registered personnel trained by the program and the National Cancer Registrars Association.15 The University of Southern California and Tokai University Institutional Review Boards exempted the current study protocol due the use of deidentified, publicly available data.
Study cohort
Women with invasive cervical cancer who had stage T1–2, N1, M0-x disease diagnosed from 1988 to 2016 who underwent hysterectomy followed by postoperative pelvic irradiation were examined. Cases prior to 1988 were excluded because of a lack of sufficient information regarding lymph node status and treatment type. Histologic types were limited to squamous, adenocarcinoma, and adenosquamous as in prior studies.3,9 Cases with stage T3–4/distant metastatic/unknown disease stage, unknown lymph node status, or no lymph node metastasis were excluded. Similarly, women treated with neoadjuvant radiotherapy, and those who received no or unknown postoperative radiotherapy, were excluded.
Clinical information
Among the eligible cases, patient demographics, tumor characteristics, treatment type, and survival outcomes were abstracted from the database. Patient demographics included age (<30, 30–39, 40–49, 50–59, 60–69, and ≥70 years), year of diagnosis (categorized as 1988–1997, 1998–2007, and 2008–2016), race/ethnicity (white, black, Hispanic, Asian, and others), registry area (West, Central, and East), and marital status (married, single, and others).
Tumor characteristics included histologic type (squamous, adenocarcinoma, and adenosquamous), tumor differentiation (well, moderate, and poor), T stage (1a, 1b, 2a, and 2b), tumor size (<2.0, 2.0–3.9, 4.0–5.9, and ≥6.0 cm), and number of sampled pelvic lymph nodes and tumor-containing lymph nodes. Treatment types included type of hysterectomy (simple/pan, radical, and not otherwise specified), pelvic lymphadenectomy (adequate vs inadequate) and concurrent chemotherapy use. Survival outcomes included follow-up time after cervical cancer diagnosis and vital status (dead or alive). Survival status in the database was externally linked to the National Death Index for validation.16
Study definition
The International Classification of Disease for Oncology, third edition, site/histology validation list and World Health Organization histological classification were used for histologic coding to identify cases as described previously.17 The American Joint Committee on Cancer staging classification schema were used for identifying T1–2 stage. Lymphadenectomy performance was based on the SEER coding for “Regional Nodes” that was introduced in 1988 and has not changed since.
Lymph node ratio (LNR) was defined as the ratio of the number of tumor-containing lymph nodes to the total number of sampled lymph nodes, and a cutoff of >7.6% was defined as high LNR because of adverse survival impacts in cervical cancer.18 Adequate lymphadenectomy was defined as ≥8 of sampled lymph node count based on the Gynecologic Oncology Group definition.19
CCRT was defined as the use of both postoperative external beam radiation and chemotherapy, and radiotherapy alone was defined as the use of external beam radiation without chemotherapy. Overall survival (OS) was defined as the time interval between the cervical cancer diagnosis and death from any cause. Women who had no survival event at the last follow-up were censored.
Statistical analysis
The Chi-square tests were used to examine differences in categorical variables between women treated with CCRT versus radiotherapy alone. A multivariable model with binary logistic regression analysis was fitted to identify the independent clinico-pathological factors associated with CCRT use. Patient demographics, tumor characteristics, and treatment types were entered in the final model to estimate the likelihood of CCRT use with adjusted odds ratios (OR) and 95% confidence intervals (CI). The Hosmer–Lemeshow test was used to assess the goodness-of-fit in the final model, and a value of >.05 was interpreted as a good-fit model.20
The National Cancer Institute’s Joinpoint Regression Program (version 4.4.0.0) was used to assess the temporal trend of CCRT use and the 5-year OS rates.21 Results were assessed with annual increment, which analyzed trends by linear segmented regression and log-transformation and was then fitted to determine the annual percent change (APC) of the each slope with 95% CI.
The propensity score–based inverse probability of treatment weighting (PS-IPTW) was used to balance the background differences between the 2 groups.22 The PS-IPTW model creates a weighted cohort that differed based on treatment type (CCRT vs radiotherapy alone) but was similar with respect to other baseline demographics. First, the PS was estimated by fitting a multivariable binary logistic regression model to predict the use of CCRT.23 All of the covariates assessed in the study including time period were entered into the model. The PS-IPTW approach assigned women who received CCRT a weight of 1/PS, and assigned those who received radiotherapy alone a weight of 1/(1-PS). We used the stabilized weight for analysis, and the threshold technique was used at the first and 99th percentile of the weight distribution.22
In the weighted model, demographics in the 2 groups were assessed for distributions, and a standardized difference (SD) of >0.20 was considered a presence of clinical size effect. The Kaplan–Meier method was used to construct survival curves. Cox proportional hazard regression models were fitted to estimate the effect size of receipt of concurrent chemotherapy on OS, expressed with a hazard ratio (HR) with 95% CI. Proportional hazard assumption was satisfied and had no interaction to time.
In a sensitivity analysis, the association of postoperative radiotherapy and OS was examined in poor-survival groups: age ≥50 years per arbitrary cutoff around the median age, T2 disease, nonsquamous histology with adenocarcinoma and adenosquamous, high LNR, and multiple node metastases per prior study.6,24-26 PS-IPTW analysis was conducted in each cohort. A quasi-experimental approach with interrupted time-series was used to assess the causal effect of the 1999 NCI alert on CCRT use, and we sought to examine whether the alert was associated with increased use of CCRT.12,13 We also tested the hypothesis that the increase in the use of CCRT use was associated with improved survival at a population level.
All statistical analyses were based on a 2-sided hypothesis, and a P value of <.05 was considered statistically significant. The Statistical Package for Social Sciences, version 24.0 (IBM SPSS, Armonk, NY) and R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for all the analyses. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were consulted to outline this observational cohort study.27
Results
Among 95,218 cases identified in the initial search, there were 2538 women who had stage T1–2 cervical cancer with pelvic lymph node metastasis who underwent primary hysterectomy and had postoperative radiotherapy between 1988 and 2016 (Figure 1). Of those, 1809 women (71.3%) received CCRT, whereas 729 women (28.7%) received radiotherapy alone.
FIGURE 1. Study schema.
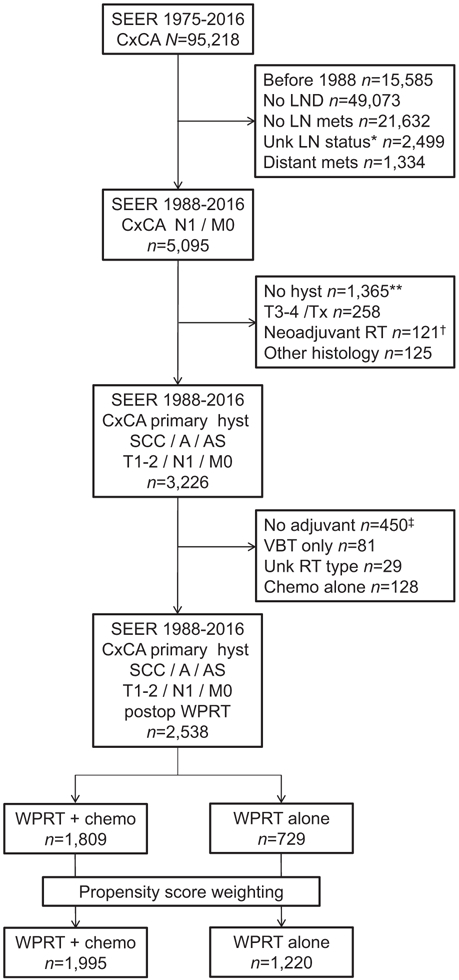
*Included 39 lymphadenectomy cases but unknown histology results. **Included 29 cases with trachelectomy, 47 cases with exenteration, and 194 cases with unknown hysterectomy status. †Included 3 cases of unknown sequence. ‡Included 1 case with unknown status. A, adenocarcinoma; AS, adenosquamous; chemo, chemotherapy; CxCA, cervical cancer; hyst, hysterectomy; LN mets, pelvic lymph node metastasis; LND, pelvic lymphadenectomy; SEER, Surveillance, Epidemiology, and End Result Program; RT, radiotherapy; SCC, squamous cell carcinoma; VBT, vaginal brachytherapy; WPRT, whole-pelvis radiotherapy.
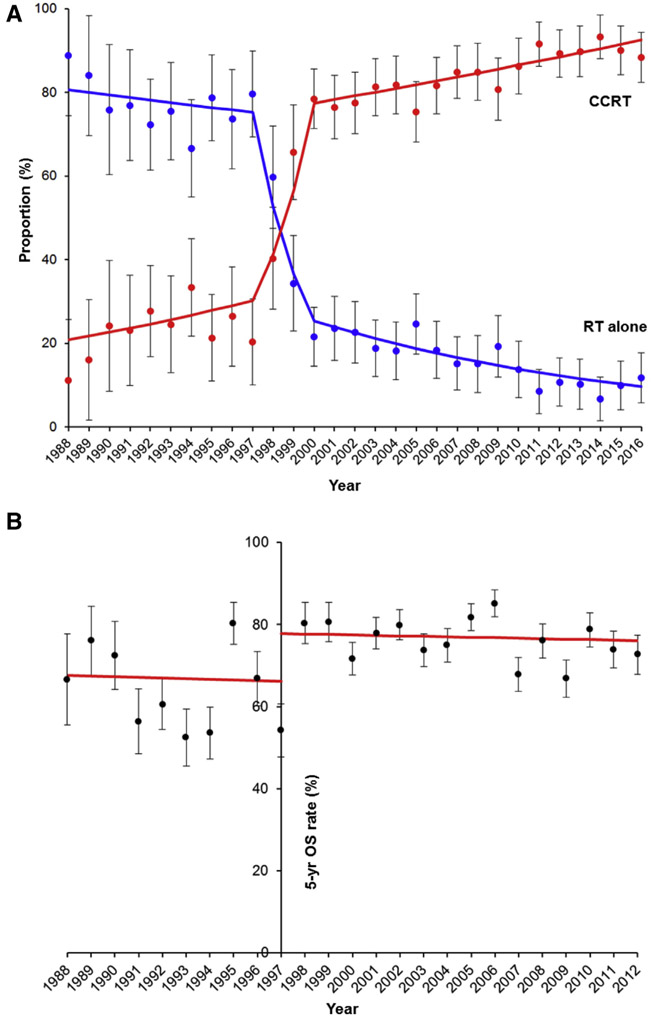
Use of CCRT was unchanged from 1988 until 1997 (APC, 4.2; 95% CI, −3.3 to 12.4; P = .267) (Figure 2A). After 1997, there was a marked treatment shift from radiotherapy alone to CCRT from 1997 to 2000 (20.7% to 78.5%; APC, 36.8; 95% CI, −0.3 to 87.3, P = .052). CCRT use continued to increase thereafter (78.5% to 88.3% between 2000 and 2016; APC, 1.1; 95% CI, 0.7–1.5, P < .001). In an interrupted time-series analysis, the 1999 NCI alert contributed to the increase in CCRT use from 65.7% to 75.5% (15% relative increase), but the rapid surge in the CCRT use had already begun a few years prior to the announcement from 20.3% to 65.7% between 1997 and 1999 (APC, 57.7; 95% CI, 11.9–122.3, P = .016) (Figure S1)
FIGURE 2. Populational trends and outcomes for concurrent chemo-radiotherapy.
A, Proportional distribution of concurrent chemo-radiotherapy (CCRT) and radiotherapy alone (RT alone) between 1988 and 2016 is shown. APC for 1988–1997 was 4.2 (95% CI, −3.3 to 12.4, P = .267); APC for 1997–2000 was 36.8 (95% CI, –0.3 to 87.6; P = .052); and APC for 2000–2016 was 1.1 (95% CI, 0.7–1.5; P < .001). B, Interrupted time-series for 5-year overall survival rate (5-yr OS rate) per population before and after 1997. The APC for 1988–1997 was −0.3 (95% CI, −5.0 to 4.8; P = .909), and APC for 1997–2012 was −0.1 (95% CI, −1.2 to 1.0, P = .776). Dots represent observed value with 95% CI. Bars represent modeled value. APC, annual percent change; CI, confidence interval.
As a cohort, the median age was 44 years (interquartile range [IQR], 36–54), and the majority of women were white (n = 1430, 56.3%). The majority of the tumors were squamous histology (n = 1695, 66.8%), poorly differentiated tumors (n = 1257, 49.5%), stage T1b disease (n = 1529, 60.2%), and had high LNR of >7.6% (n = 1482, 58.4%). Most of the women underwent adequate lymphadenectomy (n = 2192, 86.4%).
In a multivariable analysis (Table 1), women with adenocarcinoma were more likely to receive CCRT (adjusted OR, 1.331; 95% CI, 1.026–1.726) than those with squamous histology. In contrast, concurrent chemotherapy was less commonly used in older women (use of CCRT among women 40–49, 60–69, and ≥70 years of age was 50%, 67%, and 83% less compared to those <30 years of age; all, P < .01) (Table 1). In addition, residents of the central United States were 32% less likely to receive CCRT compared to those in the western United States (P = .014).
TABLE 1.
Patient demographics (n = 2538)
| RT alone |
CCRT |
||||
|---|---|---|---|---|---|
| Characteristic | n = 729 | n = 1809 | P value | Adjusted OR (95%CI) | P valuea |
| Age, y | 45 (IQR 38–56) | 43 (IQR 36–53) | <.001 | <.001b | |
| <30 | 43 (5.9%) | 131 (7.2%) | 1 | ||
| 30–39 | 181 (24.8%) | 550 (30.4%) | 0.711 (0.446–1.134) | .152 | |
| 40–49 | 234 (32.1%) | 536 (29.6%) | 0.499 (0.313–0.794) | .003 | |
| 50–59 | 124 (17.0%) | 337 (18.6%) | 0.479 (0.291–0.789) | .004 | |
| 60–69 | 89 (12.2%) | 186 (10.3%) | 0.323 (0.189–0.549) | <.001 | |
| ≥70 | 58 (8.0%) | 69 (3.8%) | 0.168 (0.091–0.310) | <.001 | |
| Year | <.001 | <.001b | |||
| 1988–1997 | 352 (48.3%) | 113 (6.2%) | 1 | ||
| 1998–2007 | 266 (36.5%) | 873 (48.3%) | 11.246 (8.516–14.852) | <.001 | |
| 2008–2016 | 111 (15.2%) | 823 (45.5%) | 27.337 (19.651–38.028) | <.001 | |
| Race/ethnicity | .009 | .459b | |||
| White | 411 (56.4%) | 1019 (56.3%) | 1 | ||
| Black | 77 (10.6%) | 127 (7.0%) | 0.713 (0.490–1.036) | .076 | |
| Hispanic | 153 (21.0%) | 460 (25.4%) | 0.915 (0.701–1.196) | .516 | |
| Asian | 76 (10.4%) | 167 (9.2%) | 0.859 (0.597–1.234) | .411 | |
| Others | 12 (1.6%) | 36 (2.0%) | 0.906 (0.413–1.991) | .806 | |
| Registry area | .003 | .006b | |||
| West | 462 (63.4%) | 1184 (65.5%) | 1 | ||
| Central | 133 (18.2%) | 238 (13.2%) | 0.681 (0.502–0.924) | .014 | |
| East | 134 (18.4%) | 387 (21.4%) | 1.192 (0.901–1.576) | .220 | |
| Marital status | .001 | .366b | |||
| Married | 364 (49.9%) | 962 (53.2%) | 1 | ||
| Single | 165 (22.6%) | 457 (25.3%) | 0.837 (0.645–1.088) | .183 | |
| Others | 186 (25.5%) | 339 (18.7%) | 0.880 (0.674–1.149) | .346 | |
| Unknown | 14 (1.9%) | 51 (2.8%) | 1.343 (0.663–2.719) | .413 | |
| Histology | .001 | .038b | |||
| Squamous | 525 (72.0%) | 1170 (64.7%) | 1 | ||
| Adenocarcinoma | 138 (18.9%) | 458 (25.3%) | 1.331 (1.026–1.726) | .031 | |
| Adenosquamous | 66 (9.1%) | 181 (10.0%) | 1.366 (0.949–1.96) | .094 | |
| Tumor differentiation | .005 | .794b | |||
| Well | 32 (4.4%) | 83 (4.6%) | 1 | ||
| Moderate | 265 (36.4%) | 726 (40.1%) | 1.208 (0.729–2.002) | .464 | |
| Poor | 362 (49.7%) | 895 (49.5%) | 1.252 (0.756–2.073) | .383 | |
| Unknown | 70 (9.6%) | 105 (5.8%) | 1.103 (0.600–2.029) | .752 | |
| T stage | <.001 | .465b | |||
| 1a | 24 (3.3%) | 61 (3.4%) | 1 | ||
| 1b | 408 (56.0%) | 1121 (62.0%) | 1.083 (0.620–1.890) | .780 | |
| 1NOS | 84 (11.5%) | 75 (4.1%) | 1.149 (0.585–2.255) | .687 | |
| 2a | 72 (9.9%) | 197 (10.9%) | 1.218 (0.646–2.296) | .543 | |
| 2b | 141 (19.3%) | 354 (19.6%) | 1.459 (0.801–2.661) | .217 | |
| 2NOS | 0 | 1 (0.1%) | Not estimable | .999 | |
| Tumor size, cm | <.001 | .546b | |||
| <2.0 | 82 (11.2%) | 224 (12.4%) | 1 | ||
| 2.0–3.9 | 230 (31.6%) | 688 (38.0%) | 1.110 (0.785–1.571) | .555 | |
| 4.0–5.9 | 180 (24.7%) | 481 (26.6%) | 1.024 (0.711–1.475) | .899 | |
| ≥6.0 | 61 (8.4%) | 175 (9.7%) | 1.201 (0.753–1.914) | .442 | |
| Unknown | 176 (24.1%) | 241 (13.3%) | 0.874 (0.593–1.289) | .497 | |
| Lymph node ratio | .002 | .842b | |||
| ≤7.6% | 263 (36.1%) | 688 (38.0%) | 1 | ||
| >7.6% | 420 (57.6%) | 1062 (58.7%) | 0.935 (0.747–1.170) | .558 | |
| Unknown | 46 (6.3%) | 59 (3.3%) | Not estimable | .999 | |
| Hysterectomy type | <.001 | .564b | |||
| Simplec | 142 (19.5%) | 465 (25.7%) | 1 | ||
| Radical | 547 (75.0%) | 1205 (66.6%) | 1.143 (0.893–1.463) | .287 | |
| NOS | 40 (5.5%) | 139 (7.7%) | 1.075 (0.696–1.660) | .745 | |
| Lymphadenectomy | .004 | ||||
| Not adequate | 69 (9.5%) | 173 (9.6%) | 1 | ||
| Adequate | 615 (84.4%) | 1577 (87.2%) | 0.879 (0.613–1.259) | .480 | |
| Unknown count | 45 (6.2%) | 59 (3.3%) | Not estimable | .999 |
Binary logistic regression model for multivariable analysis. All the listed covariates were entered in the final model. Hosmer–Lemeshow test, P = .455.
CCRT, concurrent chemo-radiotherapy; CI, confidence interval; NOS, not otherwise specified; OR, odds ratio; RT, radiotherapy.
P value for multivariable model
P value for overall interaction as a factor
Including pan-hysterectomy.
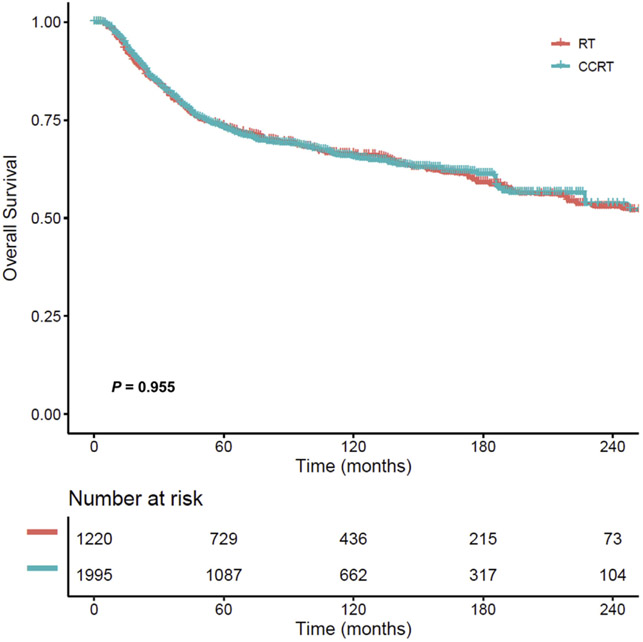
After PS-IPTW, the baseline covariates of women who received CCRT (n = 1995) compared to radiation alone (n = 1220) were well balanced (all, standardized difference, ≤0.10) (Table 2). The median follow-up time of the censored cases was 8.8 years (IQR, 4.0–14.3), and there were 1052 deaths among 3215 cases recorded during the study period. The 5-year survival rate was 73.1% (95% CI, 71.0–75.2) in women treated with CCRT vs 73.6% (95% 71.0–76.2) in those treated with RT alone. This difference was not statistically significant (HR, 1.004; 95% CI, 0.887–1.136, P = .955) (Figure 3).
TABLE 2.
Patient demographics for the PS-IPTW model (n = 3215)
| RT alone |
CCRT |
||||
|---|---|---|---|---|---|
| Characteristic | n = 1220 | n = 1995 | P value | SD (before) | SD (after) |
| Age, y | .826 | 0.1811 | 0.0374 | ||
| <30 | 69 (5.7%) | 130 (6.5%) | |||
| 30–39 | 355 (29.1%) | 586 (29.4%) | |||
| 40–49 | 368 (30.2%) | 607 (30.4%) | |||
| 50–59 | 234 (19.2%) | 364 (18.3%) | |||
| 60–69 | 130 (10.7%) | 217 (10.9%) | |||
| ≥70 | 64 (5.2%) | 90 (4.5%) | |||
| Year | .729 | 1.1292 | 0.0252 | ||
| 1988–1997 | 226 (18.5%) | 348 (17.4%) | |||
| 1998–2007 | 549 (45.0%) | 905 (45.4%) | |||
| 2008–2016 | 445 (36.5%) | 742 (37.2%) | |||
| Race/ethnicity | .942 | 0.0270 | 0.0245 | ||
| White | 702 (57.5%) | 1130 (56.6%) | |||
| Black | 104 (8.5%) | 165 (8.3%) | |||
| Hispanic | 288 (23.6%) | 488 (24.4%) | |||
| Asian | 107 (8.8%) | 175 (8.8%) | |||
| Others | 19 (1.6%) | 38 (1.9%) | |||
| Registry area | .886 | 0.0115 | 0.0012 | ||
| West | 782 (64.0%) | 1285 (64.4%) | |||
| Central | 184 (15.1%) | 288 (14.4%) | |||
| East | 255 (20.9%) | 422 (21.2%) | |||
| Marital status | .970 | 0.0940 | 0.0190 | ||
| Married | 627 (51.4%) | 1041 (52.2%) | |||
| Single | 295 (24.2%) | 480 (24.1%) | |||
| Others | 264 (21.6%) | 421 (21.1%0 | |||
| Unknown | 34 (2.8%) | 52 (2.6%) | |||
| Histology | .934 | 0.1253 | 0.0049 | ||
| Squamous | 809 (66.3%) | 1324 (66.4%) | |||
| Adenocarcinoma | 297 (24.3%) | 477 (23.9%) | |||
| Adenosquamous | 114 (9.3%) | 194 (9.7%) | |||
| Tumor differentiation | .751 | 0.1164 | 0.0326 | ||
| Well | 60 (4.9%) | 94 (4.7%0 | |||
| Moderate | 495 (40.6%) | 775 (38.8%) | |||
| Poor | 582 (47.7%) | 988 (49.5%) | |||
| Unknown | 83 (6.8%) | 138 (6.9%) | |||
| T stage | .979 | 0.0358 | 0.0253 | ||
| 1a | 39 (3.2%) | 68 (3.4%) | |||
| 1b | 747 (61.2%) | 1193 (59.8%) | |||
| 1NOS | 76 (6.2%) | 123 (6.2%) | |||
| 2a | 125 (10.2%) | 215 (10.8%) | |||
| 2b | 233 (19.1%) | 396 (19.8%) | |||
| 2NOS | 0 | 1 | |||
| Tumor size, cm | .857 | 0.2341 | 0.0284 | ||
| <2.0 | 157 (12.9%) | 238 (11.9%) | |||
| 2.0–3.9 | 446 (36.6%) | 717 (35.9%) | |||
| 4.0–5.9 | 307 (25.2%) | 513 (25.7%) | |||
| ≥6.0 | 106 (8.7%) | 192 (9.6%) | |||
| Unknown | 204 (16.7%) | 335 (16.8%) | |||
| Lymph node ratio | .433 | 0.0907 | 0.0246 | ||
| ≤7.6% | 453 (37.1%) | 749 (37.5%) | |||
| >7.6% | 705 (57.8%) | 1165 (58.4%) | |||
| Unknown | 62 (5.1%) | 82 (4.1%) | |||
| Hysterectomy type | .829 | 0.0760 | 0.0229 | ||
| Simplea | 312 (25.6%) | 493 (24.7%) | |||
| Radical | 828 (67.9%) | 1364 (68.4%) | |||
| NOS | 80 (6.6%) | 138 (6.9%) | |||
| Lymphadenectomy | .294 | 0.0825 | 0.0557 | ||
| Not adequate | 1028 (84.3%) | 1718 (86.1%) | |||
| Adequate | 131 (10.7%) | 195 (9.8%) | |||
| Unknown count | 61 (5.0%) | 82 (4.1%) |
SD ≤0.10 indicates a good-balance between the groups. A binary logistic regression test was used for the P value in the PS-IPTW model.
CCRT, concurrent chemo-radiotherapy; NOS, not otherwise specified; PS-IPTW, propensity score inverse probability of treatment weighting; RT, radiotherapy; SD, standardized difference.
Including pan-hysterectomy.
FIGURE 3. Overall survival curves based on postoperative radiotherapy type (PS-IPTW model).
Cox proportional hazard regression model for P value.
CCRT, concurrent chemo-radiotherapy; PS-IPTW, propensity score inverse probability of treatment weighting; RT, radiotherapy alone.
The association between postoperative radiotherapy type and OS was examined in various subgroups (Figure 4 and Figure S2). Among women ≥50 years of age (5-year rates, 66.0% vs 67.0%; HR, 1.107; 95% CI, 0.918–1.335; P = .288), those with nonsquamous tumors (65.1% vs 66.7%; HR, 1.061; 95% CI, 0.862–1.307; P = .576), those with stage T2 disease (66.3% vs 61.8%; HR, 1.115; 95% CI, 0.909–1.368, P = .297), those with high LNR (70.1% vs 69.3%; HR, 0.914; 95% CI, 0.785–1.065; P = .249), and those with multiple nodal metastases (69.2% vs 69.3%; HR, 0.981; 95% CI, 0.839–1.147; P = .812), CCRT was not associated with improved survival compared to radiotherapy alone. This nonsignificant association was also observed in the post–NCI alert era (75.5% vs 77.5%; HR, 1.040; 95% CI, 836–1.294; P = .724).
FIGURE 4. Forest plots for overall survival (PS-IPTW model).
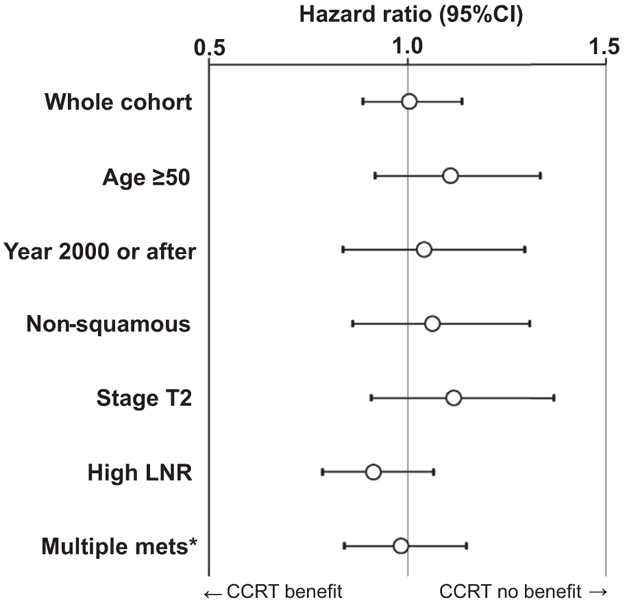
Association of postoperative radiotherapy type (CCRT vs RT) and overall survival was assessed with Cox proportional hazard regression test in the PS-IPTW model. Circles represent HR, and bars represent 95% CI. *Multiple pelvic lymph node metastases. CCRT, concurrent chemo-radiotherapy; CI, confidence interval; HR, hazard ratio; LNR, lymph node ratio; PS-IPTW, propensity score inverse probability of treatment weighting; RT, radiotherapy alone.
Finally, survival outcome was examined at a population level (Figure S3). The 5-year OS rates statistically remained unchanged during the study period (APC, 0.5; 95% CI, −0.2 to 1.3; P = .175). As the use of CCRT started increasing in 1997, we examined the populational survival rate after 1997 to examine whether increasing CCRT use resulted in improving populational survival (Figure 2B). This analysis showed no improvement in the 5-year OS rate during this time period (APC, −0.1; 95% CI, −1.2 to 1.0; P = .776).
Comment
Principal findings
These data suggest that in the late-1990s, CCRT became the mainstay of adjuvant therapy for women with early-stage cervical cancer and nodal metastases. Despite the findings of a randomized controlled trial, at the population level, CCRT did not improve overall survival compared to radiotherapy alone.
Results
The drastic treatment shift in the use of CCRT around the time of the 1999 NCI alert was an expected observation, but this practice pattern change has not previously been examined at a populational level. Our results demonstrate that the use of CCRT with radiotherapy per the NCI has been successfully implemented at a national level. Interestingly, the 1999 NCI alert accounted for only a fraction of the marked treatment shift, and the rapid adaptation of CCRT had actually started few years prior to the announcement. Increased use of CCRT in this setting may stem from the results of other trials that showed a benefit of concurrent chemotherapy for the treatment of locally advanced cervical cancer.28-32
The finding of nonsuperior survival benefit of postoperative CCRT compared to radiotherapy in high-risk early-stage cervical cancer with lymph node metastasis was unexpected. As demonstrated in a prior trial, women with high-risk early-stage cervical cancer appeared to benefit from postoperative CCRT.3 As their eligibility criteria were the presence of any 1 of 3 high-risk factors (pelvic lymph node metastasis, parametrial tumor involvement, and surgical margin involving tumor),3 our divergent results may be due to restricting the study population to only the “highest–high risk” group, those with pelvic lymph node metastasis.
However, this interpretation should be viewed with caution, as >85% of their study population had nodal metastasis. Another possibility to explain the difference between our results and their trial may be a follow-up time. At the time of early release of their results based on their special interim analysis, the median follow-up time was 3.5 years. As our study has nearly 5 years’ longer follow-up time (median, 8.8 years), this large difference in follow-up may result in differences in survival outcome between the 2 studies. It is speculated that late survival events may have occurred more, and early closure of the trial could not have captured these survival events, resulting in possible lead-time bias.
Our study validates a recent nation-wide study in Japan, in which a total of 6005 women with early-stage cervical cancer who underwent radical hysterectomy were examined. In this study, survival and recurrence patterns were similar between women treated with CCRT and radiotherapy alone.9 Our study not only provides patient-level analysis showing an absence of survival benefit, but also provides data on populational survival trends, of which the results correlated with patient-level analysis, and demonstrated again the absence of survival improvement with increasing CCRT use. Collectively, these data suggest that use CCRT for high-risk early-stage cervical cancer with lymph node metastasis maybe of modest, if any, clinical value.
Hypothesis
A possible explanation for our study results is that the presence of pelvic lymph node metastasis is a surrogate for systemic disease with microscopic tumor spread. Thus, current treatments may be inadequate in this setting because (1) the radiotherapy may not sterilize these tumors located outside of the radiation field, and (2) the chemotherapy dose and regimen for the purpose of radiosensitization may not be adequate to eliminate these tumors. This concept may be partly supported by the finding that in the women with node-positive early-stage cervical cancer who received systemic chemotherapy after surgery, there was an approximately 50% lower risk of distant recurrence compared to that in women who received CCRT.9 Thus, a treatment strategy with CCRT followed by systemic chemotherapy may need to be considered in this highest–high-risk group. As our study did not examine this combination treatment for node-positive early-stage cervical cancer, this provocative hypothesis and proposal would need to be examined further.
Study limitations
This study has several limitations. First, as inherent to the nature of retrospective studies, unmeasured bias may exist in the analysis. The presence of patient comorbidities, performance status, and treatment decision-making processes all affect the choice to initiate postoperative radiotherapy, as well as affecting survival, and were not available for analysis. Second, the mode of hysterectomy (minimally invasive vs laparotomy) and institutional surgical volume were not available in the database but can also have an impact on outcomes.33-35 Third, details of chemotherapy (regimen and administered cycle) were not available in this study, making regimen-specific analysis not feasible.
Fourth, this study examined a nearly 3-decade span of cervical cancer patients, and the inclusion of older cases weakens the applicability to current practice. Surgical technique, patient care, chemotherapeutic treatment, and radiotherapy techniques have since advanced during the study period and may have an impact on outcomes. Fifth, adverse events were not available in the database, and composite endpoint analysis together with survival was not performed. As the prior trial showed increased risks of adverse events with the addition of chemotherapy to radiotherapy,3 lack of this information limits the interpretation of our study. Sixth, recurrence information, including the anatomical site of recurrence, was also not available in the study, which did not allow for complete outcome assessment.
Seventh, due to indistinguishable surgical codes for hysterectomy, it may be possible that certain women in the simple hysterectomy group may have indeed undergone radical hysterectomy. Similarly, the use of extended-field radiotherapy was not distinguishable for radiation codes for whole-pelvis radiotherapy. Finally, the coding in SEER database limits our analysis. Prior to 2016, distinguishing clinical vs pathological stage was not applicable, and therefore it was not feasible to exclude clinical stage IIB disease in our analysis. However, as the standard treatment approach is definitive radiotherapy for clinical stage IIB disease in the United States, cases of stage T2 disease in our study population more likely represent pathological parametrial disease found in the hysterectomy specimen.
Moreover, possible misclassification of chemotherapy may have occurred in a small number of women.36 By limiting the study population to radiotherapy-administered cases, the accuracy of treatment allocation is likely high. In fact, observed results of increasing CCRT use around the time of the 1999 NCI alert support the accuracy of the data. The coding also reflects whether the chemotherapy was given for the purpose of radio-sensitization vs as systemic chemotherapy. Not included in the coding is the use of systemic chemotherapy combined with postoperative radiotherapy. This is not a standard treatment strategy in the United States, however, and such cases are presumably scant.
Clinical implications
A clinical implication of the study may be in the area of postoperative treatment management in high-risk early-stage cervical cancer with pelvic lymph node metastasis. First, further validation of our study findings would be of great benefit. Second, given the high risk of future disease recurrence, recognition of lymph node metastasis as a surrogate for systemic disease would be useful.8,9 Moreover, the unchanged 5-year OS rate over the multiple decades of this study in this highest–high risk group needs to be recognized as a call for further investigation to improve survival.
In particular, our results would suggest the need for additional systemic chemotherapy in this highest risk group.37 There is currently an ongoing phase III trial in the accrual phase (RTOG-0724), in which women with high-risk early-stage cervical cancer will receive postoperative CCRT with or without subsequent systemic chemotherapy using paclitaxel and carboplatin for 4 cycles.38 The analysis of the node-positive cases in their trial may shed light on whether additional systemic chemotherapy improves survival of this highest–high risk group.
Supplementary Material
AJOG at a Glance.
Why was this study conducted?
The presence of pelvic lymph node metastasis carries the highest association with decreased survival of the recognized surgical–pathological factors in early-stage cervical cancer. Although postoperative concurrent chemo-radiotherapy (CCRT) is the current standard treatment for high-risk early-stage cervical cancer, the effectiveness of this treatment in node-positive disease has not been completely studied.
Key findings
In an analysis of a population-based tumor registry in the United States from 1988 to 2016, there was a marked increase in the use of CCRT for surgically treated women with early-stage cervical cancer and pelvic lymph node metastasis. Women who received CCRT had overall survival similar to that in women who received radiotherapy alone.
What does this add to what is known?
Our study suggests that CCRT may not be adequate to improve survival of women with node-positive early-stage cervical cancer, suggesting the necessity of a re-examination of current practice.
Acknowledgments
Funding support was provided by Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
J.D.W. has been a Consultant to Tesaro and Clovis Oncology and has received research funding from Merck. L.D.R. has been a consultant to Quantgene. K.M. is the recipient of an honorarium (Chugai), book editorial expense (Springer), and investigator meeting attendance expense (VBL therapeutics). M.K. has been an Advisory Board member for Tesaro and GSK. The other authors report no conflict of interest.
This work was presented in part in abstract form at the 21st European Congress on Gynaecological Oncology, Athens, Greece, Nov. 2–5, 2019.
Contributor Information
Koji Matsuo, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Southern California, Los Angeles, CA; Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, CA.
David J. Nusbaum, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Southern California, Los Angeles, CA.
Hiroko Machida, Department of Obstetrics and Gynecology, Tokai University School of Medicine, Kanagawa, Japan.
Yongmei Huang, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Columbia University College of Physicians and Surgeons, New York, NY.
Varun Khetan, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Southern California, Los Angeles, CA.
Shinya Matsuzaki, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Southern California, Los Angeles, CA.
Maximilian Klar, Department of Obstetrics and Gynecology, University of Freiburg, Freiburg, Germany.
Brendan H. Grubbs, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Southern California, Los Angeles, CA.
Lynda D. Roman, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Southern California, Los Angeles, CA; Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, CA.
Jason D. Wright, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Columbia University College of Physicians and Surgeons, New York, NY.
Reference
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.NCCN. National Comprehensive Cancer Network (US) NCCN Clinical Practice Guideline in Oncology. Version 4. Cervical cancer. 2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed July 28, 2019. [Google Scholar]
- 3.Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606–13. [DOI] [PubMed] [Google Scholar]
- 4.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177–83. [DOI] [PubMed] [Google Scholar]
- 5.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 1990;38:352–7. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo K, Shimada M, Saito T, et al. Risk stratification models for para-aortic lymph node metastasis and recurrence in stage IB-IIB cervical cancer. J Gynecol Oncol 2018;29:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuo K, Mabuchi S, Okazawa M, et al. Utility of risk-weighted surgical-pathological factors in early-stage cervical cancer. Br J Cancer 2013;108:1348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YJ, Kim DY, Lee SW, et al. A postoperative scoring system for distant recurrence in node-positive cervical cancer patients after radical hysterectomy and pelvic lymph node dissection with para-aortic lymph node sampling or dissection. Gynecol Oncol 2017;144:536–40. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo K, Shimada M, Aoki Y, et al. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: systemic chemotherapy versus pelvic irradiation. Int J Cancer 2017;141:1042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv72–83. [DOI] [PubMed] [Google Scholar]
- 11.Ebina Y, Mikami M, Nagase S, et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int J Clin Oncol 2018;24:1–19. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Concurrent chemoradiation for cervical cancer Clinical announcement, February 22, 1999. Washington, DC: National Cancer Institute; 1999. [Google Scholar]
- 13.Thomas GM. Improved treatment for cervical cancer–concurrent chemotherapy and radiotherapy. N Engl J Med 1999;340:1198–200. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute’s. The Surveillance, Epidemiology, and End Results (SEER) Program. Available at: http://seer.cancer.gov/. Accessed July 28, 2019.
- 15.National Cancer Registrars Association. Available at: http://www.ncra-usa.org/i4a/pages/index.cfm?pageid=1%3e. Accessed July 28, 2019.
- 16.National Death Index. Available at: https://www.cdc.gov/nchs/ndi/. Accessed July 28, 2019.
- 17.Matsuo K, Machida H, Shoupe D, et al. Ovarian conservation and overall survival in young women with early-stage cervical cancer. Obstet Gynecol 2017;129:139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming ND, Frumovitz M, Schmeler KM, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol 2015;136:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitney CW, Spirtos N. Gynecologic Oncology Group surgical procedures manual. Philadelphia, PA: Gynecologic Oncology Group; 2009. [Google Scholar]
- 20.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997;16:965–80. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute Division of Cancer Control & Population Sciences. Joinpoint Trend Analysis Software. Available at: https://surveillance.cancer.gov/joinpoint/. Accessed July 28, 2019. [Google Scholar]
- 22.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol 2012;30:4215–22. [DOI] [PubMed] [Google Scholar]
- 24.Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol 2012;125:287–91. [DOI] [PubMed] [Google Scholar]
- 25.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Further stratification of risk groups in patients with lymph node metastasis after radical hysterectomy for early-stage cervical cancer. Gynecol Oncol 2010;117:53–8. [DOI] [PubMed] [Google Scholar]
- 26.Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol 2010;116:140–6. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154–61. [DOI] [PubMed] [Google Scholar]
- 29.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144–53. [DOI] [PubMed] [Google Scholar]
- 30.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999;340:1137–43. [DOI] [PubMed] [Google Scholar]
- 31.Thomas G, Dembo A, Ackerman I, et al. A randomized trial of standard versus partially hyperfractionated radiation with or without concurrent 5-fluorouracil in locally advanced cervical cancer. Gynecol Oncol 1998;69:137–45. [DOI] [PubMed] [Google Scholar]
- 32.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999;17:1339–48. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 2018;379:1895–904. [DOI] [PubMed] [Google Scholar]
- 34.Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med 2018;379:1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol 2019;133:1086–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Med Care 2014;54:e55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mabuchi S, Isohashi F, Yokoi T, et al. A phase II study of postoperative concurrent carboplatin and paclitaxel combined with intensity-modulated pelvic radiotherapy followed by consolidation chemotherapy in surgically treated cervical cancer patients with positive pelvic lymph nodes. Gynecol Oncol 2016;141:240–6. [DOI] [PubMed] [Google Scholar]
- 38.Chemotherapy and Pelvic Radiation Therapy With or Without Additional Chemotherapy in Treating Patients With High-Risk Early-Stage Cervical Cancer After Radical Hysterectomy. U.S. National Library of Medicine; ClinicalTrials.gov Available at: https://clinicaltrials.gov/ct2/show/NCT00980954. Accessed July 28, 2019 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.