Abstract
Objective.
To examine trends of adjuvant radiotherapy choice and to examine associations between pelvic lymphadenectomy and radiotherapy choice for women with early-stage endometrial cancer.
Methods.
The Surveillance, Epidemiology, and End Results Program was used to identify surgically treated stage I-II endometrial cancer between 1983 and 2012 (type 1 n = 79,474, and type 2 n = 25,020). Piecewise linear regression models were used to examine temporal trends of intracavitary brachytherapy (ICBT) and whole pelvic radiotherapy (WPRT) use, pelvic lymphadenectomy rate, and sampled node counts. Multivariable binary logistic regression models were used to identify independent predictors for ICBT use.
Results.
There was a significant increase in ICBT use and decrease in WPRT use during the study period. ICBT use exceeded WPRT use in 2003 for type 1 stage IA, and in 2007 for type 1 stage IB and type 2 stage IA diseases. In addition, number of sampled pelvic nodes significantly increased over time in type 1–2 stage I-II diseases (mean, 7.0–12.7 in 1988 to 15.2–17.6 in 2012, all P < 0.001). On multivariable analysis, extent of sampled pelvic nodes was significantly associated with ICBT use for type 1 cancer: adjusted-odds ratios for 1–10 and > 10 nodes versus no lymphadenectomy in stage IA (1.38/2.40), IB (2.75/6.32), and II (1.36/2.91) diseases. Similar trends were observed for type 2 cancer: adjusted-odds ratios for stage IA (1.69/3.73), IB (2.25/5.65), and II (1.36/2.19) diseases.
Conclusion.
Our results suggest that surgeons and radiation oncologists are evaluating the extent of pelvic lymphadenectomy when counseling women with early-stage endometrial cancer for adjuvant radiotherapy.
Keywords: Endometrial cancer, Early stage, Adjuvant radiotherapy, Intracavitary brachytherapy, Whole pelvic radiotherapy
1. Introduction
In 2016, endometrial cancer remains the most common gynecologic malignancy in the United States, projecting more than 60,000 newly diagnosed cases [1]. The majority of endometrial cancer is early-stage disease resulting in good prognosis, and surgery remains the mainstay of the treatment consisting of total hysterectomy and adnexectomy with possible lymphadenectomy in selected cases [2]. Patients with early-stage endometrial cancer whose tumors exhibit risk factors for disease relapse in the pelvis benefit from receiving adjuvant radiotherapy, given that vaginal cuff and pelvis are the two most common anatomical sites of recurrence in women with early-stage endometrial cancer [3,4].
Based upon mounting evidence, including a randomized study demonstrating the comparative effectiveness for local disease control and reduced radiation-related adverse effects in vaginal intracavitary brachytherapy (ICBT) compared to whole pelvic radiotherapy (WPRT) for early-stage endometrial cancer (PORTEC2 trial) [5], the most recent American Society for Radiation Oncology (ASTRO) guidelines recommend the use of ICBT as the preferred option for type 1 stage IB and type 2 stage IA endometrial cancer [6]. However, WPRT continues to be recommended for type 2 stage IB and type 1–2 stage II diseases [6]. These recommendations were also endorsed by the American Society of Clinical Oncology (ASCO) in year 2015 [7].
While ICBT irradiates the vaginal cuff, it does not sterilize the pelvic lymphatic chains. Given this important difference in treatment field for radiotherapy between ICBT and WPRT, patterns of practice for adjuvant radiotherapy may be affected by the extent of pelvic lymphadenectomy; however, the data have been missing to link this association. The aims of this study was to examine time-trends of adjuvant radiotherapy choice (ICBT versus WPRT) and to examine associations between the extent of pelvic lymphadenectomy and radiotherapy choice for women with stage I-II endometrial cancer.
2. Materials and methods
2.1. Data source and eligibility
The Surveillance, Epidemiology, and End Results (SEER) Program is a population-based tumor registry launched in 1973, supported and managed by the National Cancer Institute in the United States [8]. The SEER Program covers approximately 27.8% of the US population from 11 States and 7 areas. The SEER data are publicly available and deidentified, and the University of Southern California Institutional Review Board exempts this study. The STROBE guidelines were used to outline the observational study [9].
SEER*Stat 8.2.1 was used to extract the data set from SEER18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases (1973–2012). Cases recorded in the section for “Corpus Uteri/Uterus NOS” limited to malignancy and female sex were generated. Within the extracted dataset, stage I-II endometrial cancer cases with known pelvic lymphadenectomy status at hysterectomy and adjuvant radiotherapy information between 1983 and 2012 were included in the study. Data from 1973 to 1982 were removed due to lack of information on the surgical procedure. Women who received radiotherapy prior to the hysterectomy, uterine sarcomas, and metastatic tumors to the uterus were excluded. Variables ascertained from the database were patient demographics, tumor information, and treatment pattern.
2.2. Clinical Information
Patient demographics included age at diagnosis, calendar year at hysterectomy, ethnicity, marital status, and registration area. Tumor information included cancer stage, histologic subtype, tumor grade, tumor size, and pelvic lymph node status. Among the cases that had pelvic lymphadenectomy, number of sampled lymph nodes was abstracted. Treatment patterns included type of hysterectomy, pelvic lymphadenectomy, and postoperative radiotherapy. Adjuvant radiotherapy type was grouped as none, WPRT, and ICBT.
2.3. Definition
Type 1 endometrial cancer was defined as grade 1 and 2 endometrioid types [10]. Grade 3 endometrioid, serous, clear cell, undifferentiated, carcinosarcoma, squamous, adenosquamous, and mixed histology types were defied as type 2 endometrial cancer. Recorded cancer stage was re-classified into AJCC 7th staging classification schema. ICD-0-3 SEER site/histology validation list and the WHO histological classification were used for grouping histologic subtypes as shown previously (Table S1) [11].
2.4. Statistical consideration
The primary interest of analysis was to examine a time-trend of the use of adjuvant radiotherapy and lymphadenectomy for stage I-II endometrial cancer. The secondary interest of analysis was to examine associations of ICBT use and pelvic lymphadenectomy. On univariable analysis, Student t-test for continuous variables and chi-square test for ordinal/categorical variables were used as appropriate. Binary logistic regression models were used for multivariable analysis to determine independent contributing factors for ICBT. In this model, covariates for patient demographics, tumor characteristics, and treatment patterns were entered in the final model. Magnitudes of statistical significance were expressed with adjusted-odds ratio (aOR) and 95% confidence interval (CI).
For a time-trend analysis of adjuvant therapy and lymphadectomy per calendar year, Joinpoint Trend Software (version 4.2.0.2) provided by the National Cancer Institute was used to determine the potential changes in temporal trends [12]. Time duration was grouped every one year to provide percent frequency or mean value of collected variables. The results were analyzed with linear segmented regression test, and log-transformation was performed to determine annual percent change of the slope, expressed as annual percent change (APC) and 95%CI [13]. All statistical analyses were two-tailed, and a P-value of <0.05 was considered statistical significant. Statistical Package for Social Sciences (version 22.0, Chicago, IL, USA) was used for the analysis.
3. Results
Selection schema is shown in Fig. 1. There were 104,494 women with surgically-treated stage I-II endometrial cancer with known pelvic lymphadenectomy status, including 79,474 (76.1%) women with type 1 cancer and 25,050 (23.9%) women with type 2 cancer. Patient characteristics are shown in Table S2. The majority of women were aged >60, White, married, and Western US residents. The majority of tumors had stage IA disease and grade 1 endometrioid histology. For adjuvant radiotherapy, WPRT and ICBT use were given in 14,496 (13.9%) and 7689 (7.4%) cases, respectively. There were 56,278 (53.9%) women who underwent pelvic lymphadenectomy.
Fig. 1.
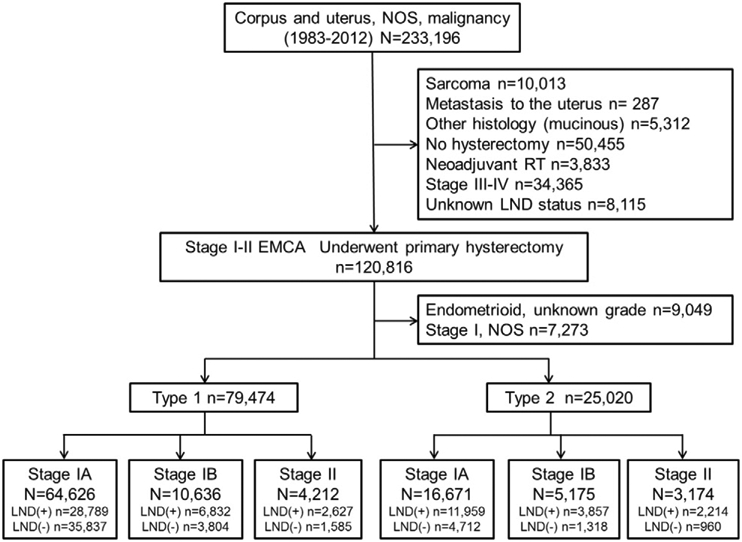
Selection schema. Abbreviations; EMCA, endometrial cancer; NOS, not otherwise specified; and LND, lymphadenectomy.
Women with type 2 cancer were more likely to be older, Black, and single compared to type 1 cancer (all, P < 0.001; Table S3). Type 2 cancer were more likely to be stage IB-II disease and had a larger tumor size compared to type 1 cancer (both, P < 0.001). Women with type 2 cancer were more likely to receive radical surgery and adjuvant radiotherapy (both, P < 0.001).
3.1. Type 1 cancer: trends of adjuvant radiotherapy and lymphadenectomy
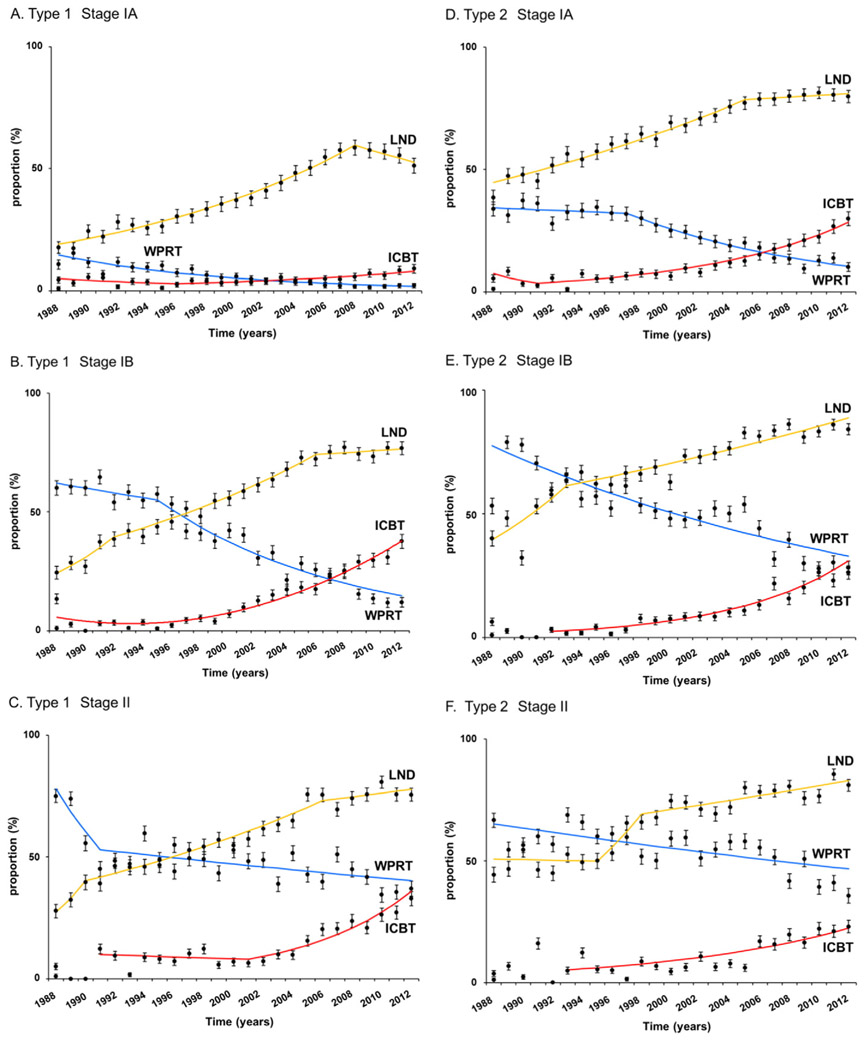
For type 1 stage IA cancer (n = 64,626), use of adjuvant radiotherapy was generally low (7.2%). During the study period, there was a significant increase in ICBT use (APC 6.7, 95%CI 4.0–9.5, P < 0.001) and a significant decrease in WPRT use (APC −8.3, 95%CI −9.6 to −7.1, P < 0.001). ICBT use rate exceeded WPRT use rate in year 2003 (Fig. 2A). In year 2012, ICBT use and WPRT use rates were 9.0% and 2.2%, respectively. Rates of women who underwent pelvic lymphadenectomy increased until year 2008 with the rate being 51.3% (APC 5.9, 95%CI 5.4–6.4, P < 0.001).
Fig. 2.
Temporal trends in treatment patterns for stage I-II endometrial cancer. Frequency of pelvic lymphadenectomy (LND, yellow line), intracavitary brachytherapy (ICBT, red line), and whole pelvic radiotherapy (WPRT, blue line) is shown for A) type I stage IA, B) type 1 stage IB, C) type I stage II, D) type II stage IA, E) type II stage IB, and F) type II stage II disease. Dots represent percent proportion with 95% confidence interval.
Similarly, among 10,636 cases of type 1 stage IB disease, ICBT use continued to increase (APC 14.1, 95%CI 12.2–16.1, P < 0.001) and WPRT use continued to decrease (APC −7.5, 95%CI −8.9 to −6.1, P < 0.001) during the study period, and ICBT use rate exceeded WPRT use rate in year 2007 (Fig. 2B). In year 2012, ICBT use and WPRT use rates were 37.5% and 11.9%, respectively. Pelvic lymphadenectomy rates also continued to increase during the study period, reaching to 76.7% in year 2012. For type 1 stage II disease (n = 4,212), ICBT use started significantly increasing in year 2001 or later (APC 14.7, 95%CI 10.1–19.4, P < 0.001), and the ICBT use rate was similar to the WPRT rate as of year 2012 (37.0% versus 39.0%, Fig. 2C).
3.2. Type 2 cancer: Trends of adjuvant radiotherapy and lymphadenectomy
Among 16,671 women with type 2 stage IA disease, ICBT use rate increased significantly after year 1993 (APC 12.3, 95%CI 10.8–13.7, P < 0.001), and WPRT use rate decreased significantly after year 1997 (APC −6.5, 95%CI −8.0 to −5.0, P < 0.001; Fig. 2D). Increasing rate of pelvic lymphadenectomy of this group reached to nearly 80% at year 2012 (79.8%). ICBT use rate exceeded WPRT use rate in year 2007. At year 2012, ICBT and WPRT use rates were 29.8% and 10.1%, respectively.
For type 2 stage IB disease (n = 5,175), increasing ICBT use rate (APC 13.5, 95%CI 11.4–15.6, P < 0.001) and decreasing WPRT use rate (APC −3.5, 95%CI −4.3 to −2.7, P < 0.001) were crossing at year 2012 (26.3% versus 28.1%; Fig. 2E). Pelvic lymphadenectomy rates exceeded 80% in year 2012 (84.1%). For type 2 stage II disease (n = 3,174), despite the high lymphadenectomy rate (year 2012, 80.9%), the whole pelvis remained the adjuvant therapy of choice (ICBT versus WPRT, 23.0% versus 35.6%; Fig. 2F).
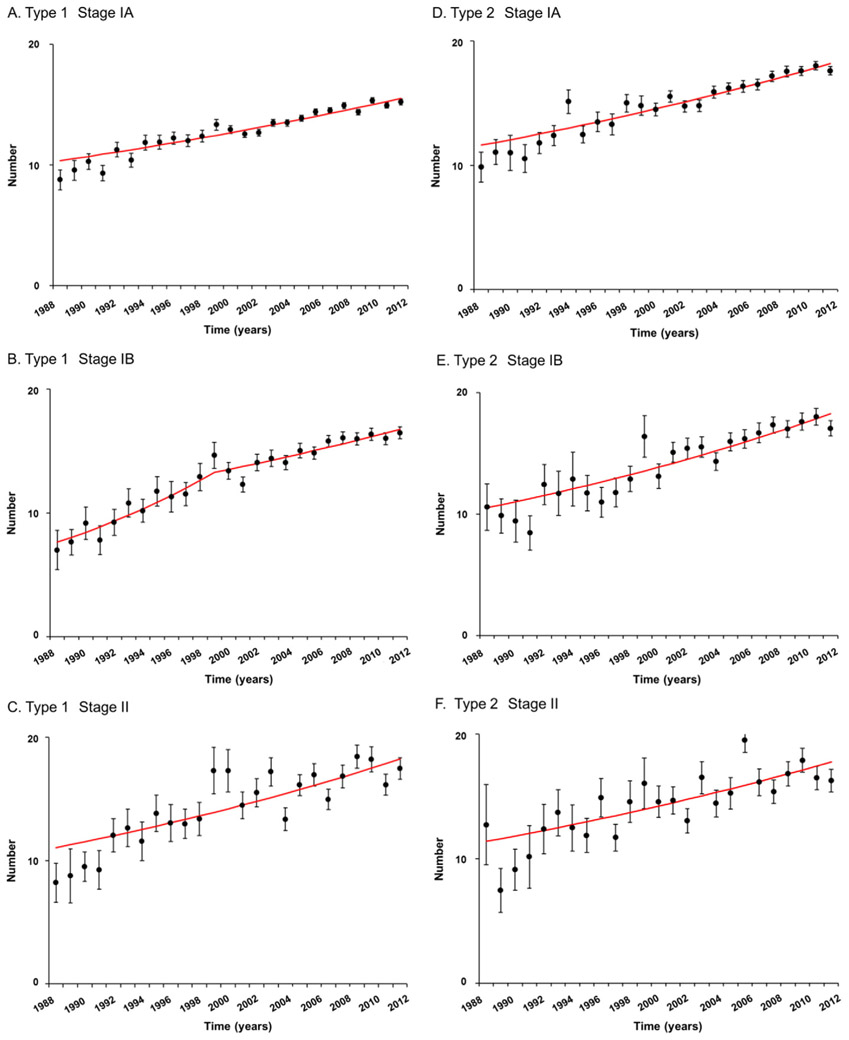
3.3. Trends in extent of lymphadenectomy for early-stage endometrial cancer
Among 56,278 women who underwent pelvic lymphadenectomy, trends in sampled number of lymph nodes were examined per histology type and stage (Fig. 3). For type 1 cancer, sampled number of pelvic lymph nodes was significantly increased between 1988 and 2012: stage IA (8.8% to 15.2%), IB (7.0% to 16.5%), and II (8.2% and 17.5%) diseases (all, P < 0.001; Fig. 3A-C). Similarly, for type 2 cancer, sampled pelvic node counts significantly increased during the study period: stage IA (9.9% to 17.6%), IB (10.6% to 17.1%), and II (12.7% to 16.3%) diseases (all, P < 0.001; Fig. 3D-F).
Fig. 3.
Trends in performance of pelvic lymphadenectomy for stage I-II endometrial cancer. Annual percent changes are: A) 1.7 (95%CI 1.4–1.9, P < 0.001), B) 5.2 between 1988 and 1999 (95%CI 3.3–7.1, P < 0.001) and 1.8 (95%CI 1.3–2.3, P < 0.001), C) 2.1 (95%CI 1.4–2.8, P < 0.001), D) 1.9 (95%CI 1.6–2.2, P < 0.001), E) 2.3 (95%CI 1.9–2.8, P < 0.001), and F) 1.9 (95%CI 1.1–2.7, P < 0.001). Bars represent mean and standard deviation. Abbreviation: CI, confidence interval.
3.4. Contributing factors for ICBT use
Among 22,185 women who received any adjuvant radiotherapy, binary logistic regression models for ICBT versus WPRT were used to identify independent contributing factor for ICBT use in each histology-specific stage group. For type 1 cancer (Table 1), White, Eastern US registry area, recent calendar year, and number of sampled pelvic nodes were the independent factors that are more likely to receive ICBT than WPRT. Specifically for sampled number of pelvic lymph nodes, higher sampled node counts were significantly associated with increased chance of receiving ICBT for stage IA (aOR for 1–10 sampled nodes and > 10 nodes compared to no pelvic lymphadenectomy, 1.36/2.40), stage IB (aOR, 2.75/7.62), and stage II (aOR, 1.36/2.91) diseases (all, P < 0.05).
Table 1.
Independent factors for intracavitary brachytherapy use in type 1 endometrial cancer.
| Characteristic | No. | Stage IA aOR (95%CI) |
P-value | No. | Stage IB aOR (95%CI) |
P-value | No. | Stage II aOR (95%CI) |
P-value |
|---|---|---|---|---|---|---|---|---|---|
| Age (continuous) | 4669 | 0.99 (0.98–1.00) | 0.09 | 5085 | 1.01 (1.00–1.02) | 0.024 | 2425 | 0.99 (0.99–1.00) | 0.13 |
| Ethnicity | |||||||||
| White | 3825 | 1 | 4299 | 1 | 1878 | 1 | |||
| Black | 299 | 0.68 (0.53–0.89) | 0.004 | 188 | 0.66 (0.46–0.96) | 0.031 | 144 | 0.77 (0.49–1.19) | 0.24 |
| Hispanic | 332 | 0.60 (0.46–0.78) | <0.001 | 351 | 0.70 (0.52–0.93) | 0.015 | 224 | 0.56 (0.38–0.83) | 0.004 |
| Others | 213 | 0.81 (0.59–1.12) | 0.21 | 247 | 0.85 (0.61–1.19) | 0.34 | 179 | 0.75 (0.49–1.15) | 0.19 |
| Marital status | |||||||||
| Single | 1863 | 1 | 2325 | 1 | 1116 | 1 | |||
| Others | 2646 | 0.99 (0.87–1.14) | 0.94 | 2601 | 1.70 (1.01–1.35) | 0.034 | 1242 | 1.03 (0.84–1.27) | 0.79 |
| Unknown | 160 | 0.90 (0.64–1.29) | 0.57 | 159 | 1.42 (0.96–2.10) | 0.08 | 67 | 0.95 (0.50–1.77) | 0.86 |
| Registry area | |||||||||
| West | 1471 | 1 | 2176 | 1 | 1111 | 1 | |||
| Central | 1016 | 1.41 (1.17–1.69) | <0.001 | 1301 | 0.67 (0.55–0.82) | <0.001 | 581 | 1.05 (0.79–1.40) | 0.69 |
| East | 2182 | 2.97 (2.54–3.47) | <0.001 | 1608 | 1.38 (1.17–1.63) | <0.001 | 733 | 1.40 (1.10–1.78) | 0.007 |
| Year at diagnosis | |||||||||
| 1988–1999 | 1560 | 1 | 1344 | 1 | 757 | 1 | |||
| 2000–2009 | 2209 | 1.64 (1.41 (1.92) | <0.001 | 2722 | 4.29 (3.27–5.64) | <0.001 | 1217 | 1.84 (1.33–2.54) | <0.001 |
| 2010–2012 | 900 | 4.25 (3.44–5.24) | <0.001 | 1019 | 16.4 (12.2–22.0) | <0.001 | 451 | 4.15 (2.92–5.89) | <0.001 |
| Surgery type | |||||||||
| Total/pan/simple hyst | 3933 | 1 | 4364 | 1 | 1918 | 1 | |||
| MRH/RH | 536 | 0.51 (0.41–0.65) | <0.001 | 527 | 0.64 (0.48–0.86) | 0.003 | 413 | 1.10 (0.78–1.54) | 0.59 |
| Others | 200 | 0.73 (0.64–0.99) | 0.045 | 194 | 0.86 (0.60–1.22) | 0.39 | 94 | 1.55 (0.96–2.51) | 0.08 |
| Number of LND | |||||||||
| 0 node | 2011 | 1 | 1856 | 1 | 841 | 1 | |||
| 1–10 nodes | 1130 | 1.38 (1.17–1.62) | <0.001 | 1426 | 2.75 (2.27–3.34) | <0.001 | 640 | 1.36 (1.01–1.84) | 0.046 |
| >10 nodes | 1389 | 2.40 (2.03–2.84) | <0.001 | 1626 | 6.32 (5.24–7.62) | <0.001 | 880 | 2.91 (2.21–3.82) | <0.001 |
Binary logistic regression models for multivariable analysis. All listed covariates were entered in the final models Significant P-values are emboldened. Abbreviations: No., number; aOR, adjusted-odds ratio; CI, confidence interval; MRH, modified radical hysterectomy; RH, radical hysterectomy; hyst, hysterectomy; and LND, pelvic lymph node dissection.
For type 2 cancer (Table 2), Eastern residents in the United States, recent calendar year, and sampled pelvic node number were associated with ICBT use. Similar to type 1 cancer, extent of sampled pelvic nodal counts were associated with increased chance of ICBT use, although it did not reach statistical significance in stage II disease: aOR for 1–10 sampled nodes and > 10 nodes compared to no pelvic lymphadenectomy, 1.69/3.73 for stage IA, 2.25/5.65 for stage IB, and 1.36/2.19 for stage II disease, respectively. In each cancer type, stage IB disease with pelvic nodal counts > 10 had the largest magnitude of significance for likelihood of ICBT use: type 1 aOR 6.32 and type 2 aOR 5.65.
Table 2.
Independent factors for intracavitary brachytherapy use in type 2 endometrial cancer.
| Characteristic | No. | Stage IA aOR (95%CI) |
P-value | No. | Stage IB aOR (95%CI) |
P-value | No. | Stage II aOR (95%CI) |
P-value |
|---|---|---|---|---|---|---|---|---|---|
| Age (continuous) | 5184 | 1.00 (0.99–1.01) | 0.75 | 2914 | 1.01 (0.99–1.02) | 0.12 | 1908 | 1.01 (0.99–1.02) | 0.19 |
| Ethnicity | |||||||||
| White | 3860 | 1 | 2337 | 1 | 1321 | 1 | |||
| Black | 591 | 0.74 (0.60–0.91) | 0.004 | 192 | 0.65 (0.42–0.99) | 0.045 | 250 | 0.96 (0.65–1.41) | 0.82 |
| Hispanic | 419 | 0.63 (0.49–0.80) | <0.001 | 210 | 0.82 (0.55–1.22) | 0.33 | 192 | 0.64 (0.41–1.02) | 0.06 |
| Others | 314 | 0.73 (0.56–0.97) | 0.028 | 175 | 0.70 (0.43–1.06) | 0.09 | 145 | 0.81 (0.47–1.38) | 0.44 |
| Marital status | |||||||||
| Single | 2184 | 1 | 1355 | 1 | 920 | 1 | |||
| Others | 2818 | 1.18 (1.04–1.35) | 0.013 | 1485 | 1.01 (0.82–1.24) | 0.96 | 935 | 0.99 (0.76–1.30) | 0.95 |
| Unknown | 182 | 1.32 (0.93–1.86) | 0.17 | 74 | 1.20 (0.65–2.20) | 0.56 | 53 | 1.08 (0.51–2.27) | 0.85 |
| Registry area | |||||||||
| West | 2479 | 1 | 1438 | 1 | 972 | 1 | |||
| Central | 1037 | 0.80 (0.67–0.96) | 0.018 | 698 | 0.78 (0.58–1.04) | 0.09 | 413 | 0.78 (0.53–1.14) | 0.20 |
| East | 1668 | 1.69 (1.46–1.96) | <0.001 | 778 | 1.78 (1.41–2.26) | <0.001 | 523 | 1.85 (1.38–2.48) | <0.001 |
| Year at diagnosis | |||||||||
| 1988–1999 | 1311 | 1 | 772 | 1 | 613 | 1 | |||
| 2000–2009 | 2465 | 2.54 (2.07–3.13) | <0.001 | 1583 | 2.71 (1.79–4.10) | <0.001 | 412 | 2.03 (1.34–3.07) | 0.001 |
| 2010–2012 | 1408 | 7.73 (6.18–9.66) | <0.001 | 559 | 9.26 (6.01–14.3) | <0.001 | 68 | 5.76 (3.75–8.87) | <0.001 |
| Surgery type | |||||||||
| Total/pan/simple hyst | 4151 | 1 | 2435 | 1 | 1428 | 1 | |||
| MRH/RH | 846 | 0.68 (0.55–0.84) | <0.001 | 380 | 0.60 (0.39–0.94) | 0.024 | 412 | 0.98 (0.67–1.42) | 0.91 |
| Others | 187 | 0.54 (0.39–0.75) | <0.001 | 99 | 1.10 (0.65–1.84) | 0.73 | 68 | 0.92 (0.48–1.77) | 0.81 |
| Number of LND | |||||||||
| 0 node | 1018 | 1 | 689 | 1 | 484 | 1 | |||
| 1–10 nodes | 1419 | 1.69 (1.37–2.08) | <0.001 | 816 | 2.25 (1.68–3.58) | <0.001 | 542 | 1.36 (0.91–2.03) | 0.13 |
| >10 nodes | 2547 | 3.73 (3.08–4.53) | <0.001 | 1271 | 5.65 (3.97–8.04) | <0.001 | 783 | 2.19 (1.51–3.18) | <0.001 |
Binary logistic regression models for multivariable analysis. All listed covariates were entered in the final models Significant P-values are emboldened. Abbreviations: No., number; aOR, adjusted-odds ratio; CI, confidence interval; MRH, modified radical hysterectomy; RH, radical hysterectomy; hyst, hysterectomy; and LND, pelvic lymph node dissection.
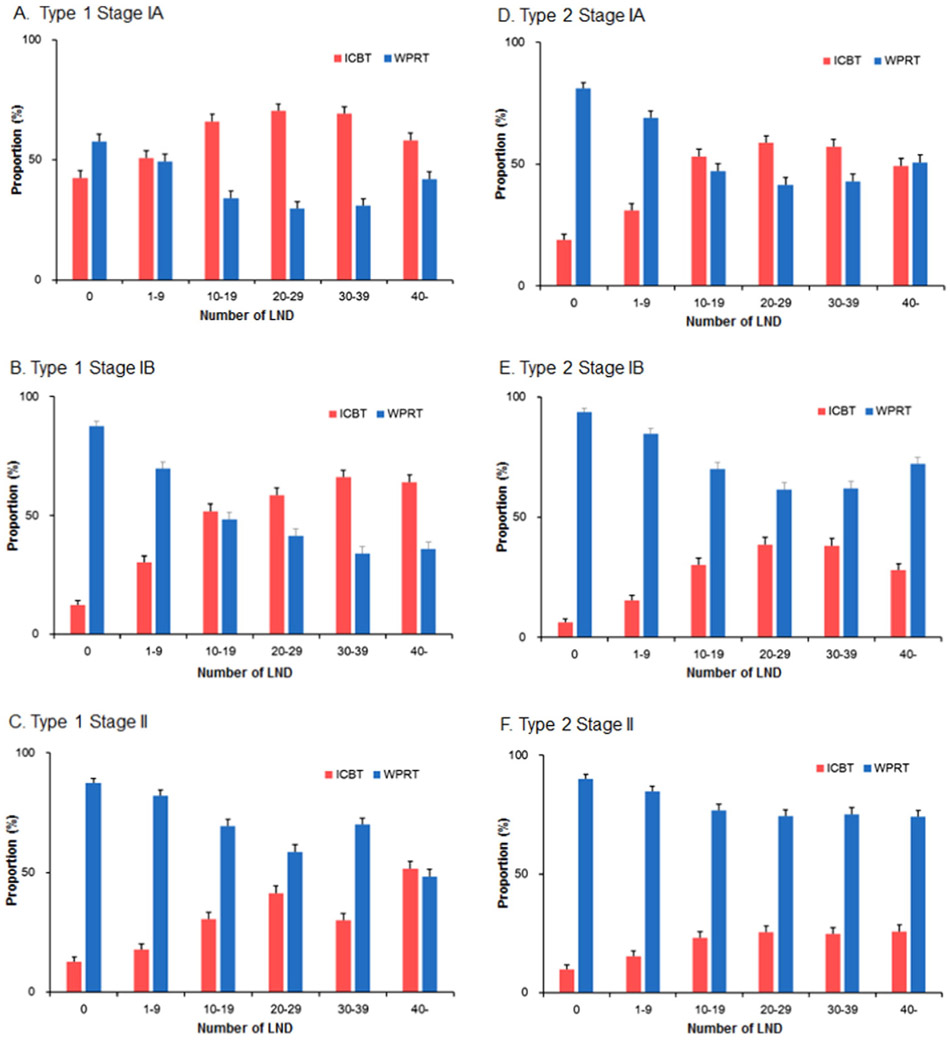
3.5. Association of sampled node counts and ICBT use
Proportion of ICBT use among women who received either ICBT or WPRT was examined based on the extent of sampled pelvic node counts (Fig. 4). For type 1 cancer, ICBT use rates clearly exceeded WPRT use when ≥10 nodes were removed in stage IA disease and when ≥20 nodes were removed in stage IB disease (Fig. 4A-B). For stage II disease, although there is a trend of increasing ICBT use in larger sampled node counts, it did not clearly exceed WPRT use (Fig. 4C). For type 2 cancer, ICBT use exceeded WPRT use when ≥20 nodes were sampled in stage IA disease (Fig. 4D). For stage IB and II diseases, although proportion of ICBT use increased as paralleled to increasing sampled node counts, the majority of adjuvant radiotherapy mode remained WRPT (Fig. 4E-F).
Fig. 4.
Radiotherapy choice bases on extent of pelvic lymphadenectomy. Bars represent proportion and 95% confidence interval. Among women who received adjuvant radiotherapy, proportion of intracavitary brachytherapy (ICBT) and whole pelvic radiotherapy (WPRT) are shown based on sampled nodes for pelvic lymphadenectomy (LND): A) type I stage IA, B) type I stage IB, C) type I stage II, D) type II stage IA, E) type II stage IB, and F) type II stage II.
4. Discussion
A salient finding of the study is that ICBT is becoming a more common modality for adjuvant radiotherapy in women with early-stage endometrial cancer, replacing WPRT in stage I disease. In addition, this study showed that extent of pelvic lymphadenectomy has been changed during the past decades, demonstrating a significant increase in sampled number of pelvic lymph nodes that were indicated for women with early-stage endometrial cancer. Most importantly, the increasing use of ICBT and the increasing number of pelvic nodal counts were correlated together, and this study found an independent association that patients are more likely to receive ICBT over WPRT if pelvic lymph nodes are adequately sampled during the indicated pelvic lymphadenectomy for early-stage endometrial cancer.
As shown in our study, recent years have witnessed a significant increase in the use of ICBT as the choice for adjuvant radiotherapy for early-stage endometrial cancer in the United States [14,15]. While this increase may solely reflect the ASTRO guidelines for the recommendation of adjuvant radiotherapy as well as the results from PORTEC2 trial demonstrating equivalent vaginal recurrence rates (5-year cumulative rates, 1.8% versus 1.6%) between ICBT and WPRT performed for women with high-intermediate risk endometrial cancer without lymphadenectomy [5], it has been not well known if the extent of pelvic lymphadenectomy is a potential cause of this increase in ICBT use.
The National Comprehensive Cancer Network (NCCN) guidelines recommend additional lymphadenectomy during hysterectomy-based surgical treatment for the women with risk factors for lymph node metastasis [16], and there has been a trend of increase in pelvic lymphadenectomy rate until recent years in early-stage endometrial cancer [17,18]. In addition, among women who underwent pelvic lymphadenectomy for early-stage endometrial cancer, the number of sampled nodes has been increased in the past years [17]. Therefore, it is speculated that surgeons and radiation oncologists may prefer to choose ICBT over WPRT as the mode of adjuvant radiotherapy for early-stage endometrial cancer if pelvic lymphadenectomy was adequately performed, given that the main difference between ICBT and WPRT is that the former covers only vaginal cuff whereas the latter covers other pelvic areas including lymphatic chains.
The recent evidence-based guidelines recommend the ICBT use over WPRT in type 1 stage IB and type 2 stage IA diseases [6]. In our study, we have found that the ICBT use eminently exceeded WPRT use when ≥20 nodes were sampled for pelvic lymphadenectomy in both groups (Fig. 4B and D). Conversely, when pelvic lymphadenectomy was not performed or not adequately performed (1–9 nodes sampled), the vast majority of such cases received WPRT. Among the women whom 10–19 pelvic nodes were sampled, ICBT use was higher than WPRT use but the difference was small. A recent study examining the National Cancer Data Base reported the possible overuse of WPRT in stage I endometrial cancer [19]. Specifically, their study showed that women who did not undergo pelvic lymphadenectomy were more likely to receive WPRT over ICBT compared to those who underwent pelvic lymphadenectomy (relative risk, 2.32). Our study is more specific and informative in that we showed the possible overuse of WPRT even in women who underwent pelvic lymphadenectomy with low nodal counts.
As supported by the current guideline recommendations [6,16], the majority of women with type 2 stage IB and type 1–2 stage II diseases received WPRT rather than ICBT for adjuvant radiotherapy in our study (Fig. 4C and E-F). However, even in these high-risk groups for pelvic recurrence, there was an increasing trend of ICBT use as paralleling to the increase in sampled pelvic node counts, and the ICBT use rate is nearly the same as the WPRT use rate for type 1 stage II and type 2 stage IB diseases in year 2012 (Fig. 2C and E). Based on this time-trend of ICBT use in the past decades for the two groups, further study will be warranted to examine if the use of ICBT may exceed WPRT in this subgroup.
This study has a number of limitations. First, this is a retrospective study, and there might be a possible confounding factor that was missing in the database. For example, the exact reason for decision making process for allocating ICBT versus WPRT was not able to abstract. Second, there was no database record for how the lymphatic tissues were sectioned for counting the number of sampled nodes. Because this study spans a few decades, there may be a possibility that the standard for tissue sectioning for lymph nodes may have changed. Third, The SEER Program does not have information for chemotherapy. Systemic chemotherapy has played an increasing role in adjuvant treatment after surgery of women with serous and clear cell carcinoma of the endometrium, and the use of chemotherapy might have impacted the choice of radiation modality in such patients [6]. This study was not able to factor this information into the analysis. Lastly, we were note able to assess other tumor factors such as presence of lymphovascular space invasion that can affect for choosing the radiation modality in this study.
A weakness of the study is that this study did not examine the type of ICBT (high-dose versus low-dose). There may be a possibility that a recent increase in the use of ICBT in the United States may be due to the increased use of high-dose ICBT that is known to reduce treatment costs compared to low-dose counterpart [20]. Similarly, this study did not examine the details of WPRT and it is not known what proportion of women received intensity-modified radiotherapy. Strength of the study is that this study examined a large-scale size with long-term follow-up. Time-trend analyses identified the turning and reflection point that the radiation modalities changed.
Our results strongly suggest that clinicians take the extent of pelvic lymphadenectomy into account when deciding the radiotherapy type for adjuvant therapy in early-stage endometrial cancer. Recently, there are two randomized controlled trials to examine the adjuvant radiotherapy for early-stage endometrial cancer (NCT00411138 and NCT00807768) [21,22]. In one trial, efficacy for adjuvant therapy was compared between ICBT combined with systemic chemotherapy and WPRT (including intensity-modified radiotherapy) for women with high-intermediate risk endometrial cancer [22]. This trial which strongly encourages patients to undergo pelvic and/or para-aortic lymphadenectomy showed more pelvic recurrence in the ICBT with chemotherapy arm compared to the WPRT arm in the preliminary analysis (6.5% versus 0.7%) but the 2-year overall survival rates were similar between the two arms (92% versus 93%) [22]. It is possible that the extent of lymphadenectomy could impact the pelvic recurrence pattern in this trial, but there will be other confounding factors such as histology types and demographics which could also play a role in the difference in pelvic recurrence. The other trial examined effectiveness and adverse effects of WPRT with or without chemotherapy for women with high-risk endometrial cancer, and survival analysis are currently undergoing [21]. At this point, the extent of lymphadenectomy has not as of yet been reported in these studies, but these trials will provide the role of lymphadenectomy in the treatment choice for adjuvant radiotherapy in type 2 stage IB and type 1–2 stage II endometrial cancer.
There are currently multiple ongoing trials examining the efficacy of sentinel lymph node sampling for endometrial cancer. Potential benefits of the procedure are to reduce the risk of lower extremity lymphedema and to improve sensitivity for detecting stage IIIC1 disease [23-25]. The impact on choice of radiation modality (ICBT versus WPRT) for women with endometrial cancer who undergo sentinel lymph node sampling is undetermined and merits further study.
Multi-disciplinary approach is suggested for decision making process when adjuvant radiotherapy is considered for treatment plan in women with early-stage endometrial cancer, and risks and benefits for each radiotherapy modality are suggested to discuss with patients [26]. Our results, in this setting, have a value to provide useful information to both patients and care providers in this process.
Supplementary Material
HIGHLIGHTS.
ICBT is replacing WPRT in adjuvant radiotherapy for stage I endometrial cancer
Pelvic node counts among lymphadenectomy cases have been significantly increased
Higher sampled pelvic node counts were associated with increased chance of ICBT use
Footnotes
Disclosure statement
There is no conflict of interest in all authors.
Financial support
Ensign Endowment for Gynecologic Cancer Research (K.M.)
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2016.12.012.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2016, CA Cancer J. Clin 66 (2016) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ, Contemporary management of endometrial cancer, Lancet 379 (2012) 1352–1360. [DOI] [PubMed] [Google Scholar]
- [3].Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG, A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic oncology group study, Gynecol. Oncol 92 (2004) 744–751. [DOI] [PubMed] [Google Scholar]
- [4].Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, De Winter KA, Lutgens LC, van den Bergh AC, van de Steen-Banasik E, Beerman H, van Lent M, Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC study group. Post operative radiation therapy in endometrial carcinoma, Lancet 355 (2000) 1404–1411. [DOI] [PubMed] [Google Scholar]
- [5].Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A, Kroese MC, van Bunningen BN, Ansink AC, van Putten WL, Creutzberg CL, Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial, Lancet 375 (2010)816–823. [DOI] [PubMed] [Google Scholar]
- [6].Klopp A, Smith BD, Alektiar K, Cabrera A, Damato AL, Erickson B, Fleming G, Gaffney D, Greven K, Lu K, Miller D, Moore D, Petereit D, Schefter T, Small W Jr., Yashar C, Viswanathan AN, The role of postoperative radiation therapy for endometrial cancer: executive summary of an American Society for Radiation Oncology evidence-based guideline, Pract Radiat. Oncol 4 (2014) 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meyer LA, Bohlke K, Powell MA, Fader AN, Franklin GE, Lee LJ, Matei D, Coallier L, Wright AA, Postoperative radiation therapy for endometrial cancer: American Society of Clinical Oncology clinical practice guideline endorsement of the American Society for Radiation Oncology evidence-based guideline, J. Clin. Oncol 33 (2015) 2908–2913. [DOI] [PubMed] [Google Scholar]
- [8].http://seer.cancer.gov/ (accessed 11/16/2015).
- [9].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, Muderspach LI, Roman LD, Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome, Obstet Gynecol. 125 (2015) 424–433. [DOI] [PubMed] [Google Scholar]
- [11].Matsuo K, Machida H, Shoupe D, Melamed A, Muderspach LI, Roman LD, Wright JD, Ovarian conservation and overall survival in young women with early-stage low-grade endometrial cancer, Obstet. Gynecol 128 (2016) 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].http://surveillance.cancer.gov/joinpoint> (accessed 11/16/2015).
- [13].Kim HJ, Fay MP, Feuer EJ, Midthune DN, Permutation tests for joinpoint regression with applications to cancer rates, Stat. Med 19 (2000) 335–351. [DOI] [PubMed] [Google Scholar]
- [14].Modh A, Ghanem AI, Burmeister C, Rasool N, Elshaikh MA, Trends in the utilization of adjuvant vaginal brachytherapy in women with early-stage endometrial carcinoma: results of an updated period analysis of SEER data, Brachytherapy 15 (2016) 554–561. [DOI] [PubMed] [Google Scholar]
- [15].Patel MK, Cote ML, Ali-Fehmi R, Buekers T, Munkarah AR, Elshaikh MA, Trends in the utilization of adjuvant vaginal cuff brachytherapy and/or external beam radiation treatment in stage I and II endometrial cancer: a surveillance, epidemiology, and end-results study, Int. J. Radiat. Oncol. Biol. Phys 83 (2011) 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 9/ 24/2016).
- [17].Melamed A, Rauh-Hain JA, Clemmer JT, Diver EJ, Hall TR, Clark RM, Uppal S, Goodman A, Boruta DM 2nd., Changing trends in lymphadenectomy for endometrioid adenocarcinoma of the endometrium, Obstet. Gynecol 126 (2015) 815–822. [DOI] [PubMed] [Google Scholar]
- [18].Wright JD, Huang Y, Burke WM, Tergas AI, Hou JY, Hu JC, Neugut AI, Ananth CV, Hershman DL, Influence of lymphadenectomy on survival for early-stage endometrial cancer, Obstet. Gynecol 127 (2015) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wright JD, Margolis B, Hou JY, Burke WM, Tergas AI, Huang Y, Hu JC, Ananth CV, Neugut AI, Hershman DL, Overuse of external beam radiotherapy for stage I endometrial cancer, Am.J. Obstet. Gynecol 215 (75) (2016) e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pinilla J, Cost minimization analysis of high-dose-rate versus low-dose-rate brachytherapy in endometrial cancer. Gynecology tumor group, Int. J. Radiat. Oncol. Biol. Phys 42 (1998) 87–90. [DOI] [PubMed] [Google Scholar]
- [21].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, Colombo A, Fyles A, Baron MH, Kitchener HC, Nijman HW, Kruitwagen RF, Nout RA, Verhoeven-Adema KW, Smit VT, Putter H, Creutzberg CL, Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): an open-label, multicentre, randomised, phase 3 trial, Lancet Oncol. 17 (2016) 1114–1126. [DOI] [PubMed] [Google Scholar]
- [22].McMeekin DS, Filiaci VL, Aghajanian C, Cho J, Kim JW, DiSilvestro PA, O'Malley D, Rutherford TJ, Van Le L, Randall ME, A randomized phase III trial of pelvic radiation therapy (PXRT) versus vaginal cuff brachytherapy followed by pacli-taxel/carboplatin chemotherapy (VCB/C) in patients with high risk (HR), early stage endometrial cancer (EC): a Gynecologic Oncology Group trial, 45th Annual Meeting on Women's Cancer, Tampa, FL, March 22–25, 2014. [Google Scholar]
- [23].Cormier B, Rozenholc AT, Gotlieb W, Plante M, Giede C, Sentinel lymph node procedure in endometrial cancer: a systematic review and proposal for standardization of future research, Gynecol. Oncol 138 (2015) 478–485. [DOI] [PubMed] [Google Scholar]
- [24].Plante M, Touhami O, Trinh XB, Renaud MC, Sebastianelli A, Grondin K, Gregoire J, Sentinel node mapping with indocyanine green and endoscopic near-infrared fluorescence imaging in endometrial cancer. A pilot study and review of the literature, Gynecol. Oncol 137 (2015) 443–447. [DOI] [PubMed] [Google Scholar]
- [25].Kang S, Yoo HJ, Hwang JH, Lim MC, Seo SS, Park SY, Sentinel lymph node biopsy in endometrial cancer: meta-analysis of 26 studies, Gynecol. Oncol 123 (2011) 522–527. [DOI] [PubMed] [Google Scholar]
- [26].Harkenrider MM, Block AM, Siddiqui ZA, Small W Jr., The role of vaginal cuff brachytherapy in endometrial cancer, Gynecol. Oncol 136 (2015) 365–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.