Abstract
Various cancer therapies have been developed, but a tumor recurrence with incomplete tumor killing and remained tumor cells/tissues are frequent in the monotherapies. Herein we report a nano-bio therapeutic emulsion formulated with multi-functional nanoscintillators and anaerobic C. novyi-NT spores for synergistic image-guided combinational cancer therapy. MRI visible nanoscintillators (NSs) were synthesized with a NaGdF4:Tb,Ce@NaGdF4 core/shell structure for an image-guided X-ray photodynamic therapy (PDT) of the normoxic peripheral tumor. An anaerobic oncolytic bacterium (Clostridium novyi-NT) therapy was combined to treat the hypoxic central tumor tissues. PS-coated NSs (PS-NSs) and C. novyi-NT spores were emulsified with clinically available ethiodized oil (Lipiodol™) to be nano-bio therapeutic emulsion and injected into the tumor with CT-image guidance. The distribution of nano-bio therapeutic emulsion, including PS-NSs and anaerobic C. novyi-NT spores in the tumor site, was confirmed by both X-ray and T1-weighted MR imaging. Following the image-guided X-ray PDT and anaerobic C. novyi-NT combination treatment, apoptotic cell death in cancer tissues, including both peripheral and central tumor region were significantly higher than in the control groups. This combination therapy approach using a nano-bio therapeutic emulsion is expected to overcome the limitations of conventional cancer therapy, resulting in increased cancer-therapeutic efficacy.
Keywords: Nano-bio emulsion, X-ray photodynamic therapy (PDT), Bacteriolytic therapy, Scintillating nanoparticles, Tumor Microenvironment (TME)
Graphical Abstract
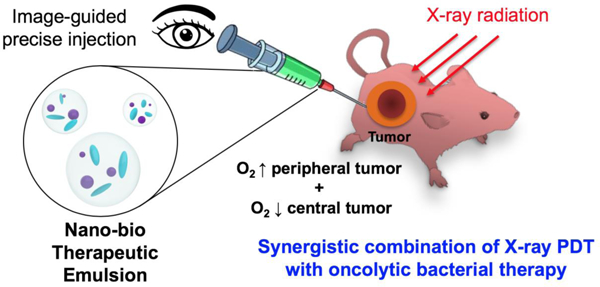
We developed a tumor microenvironment targeting nano-bio therapeutic emulsion formulated with multi-functional nanoscintillators and anaerobic C. novyi-NT spores for synergistic combinational cancer therapy. Nano-bio therapeutic emulsion was delivered to tumor sites under CT-image guidance, and effectively treated the normoxic peripheral and hypoxic central tumor region.
1. Introduction
Over the years, many innovative cancer therapies have shown excellent initial efficacy[1], but incomplete tumor killing and remaining cancer cells/tissues in the treated primary region are often developed to tumor recurrence in a short term.[2] Carefully designed combination therapies have been shown to be an effective tumor treatment option minimizing remained cancer cells or tissues. Investigating various combination therapies are currently great of interest for improving therapeutic efficacy.
Photodynamic therapy (PDT) is a relatively new and robust form of cancer therapy. PDT utilizes photosensitizers (PSs) that are activated by light in the presence of oxygen, generating cytotoxic reactive oxygen species (ROS). Despite the promise of PDT cancer therapy, insufficient local distribution of systemic intravenously (IV) or orally administered PS[3] and the shallow tissue penetration of the light limit its broad clinical application. Accordingly, PDT has mainly been used to treat lesions located just under the skin or in the lining of organs that can be reached with a light source. Recently, PSs that can be activated with near-infrared (NIR) light have been developed. However, even in the NIR range, light can travel less than 5 cm in tissue.[4] Consequently, tumor recurrence is often observed at the primary endpoint of PDT therapy in the clinic. New PDT approaches in which advanced PSs are combined with other treatment strategies such as chemotherapeutics,[5] thermal therapy,[6] radiotherapy,[7] oncolytic virus[8] or immunotherapy[7b, 9] are required to improve the therapeutic effect of PDT.
Bacteriolytic therapy is a cancer treatment with a long history that uses attenuated oncolytic bacteria.[10] Clostridium novyi (C. novyi) is rendered non-pathogenic by a selection of a clone without the major toxin (α-toxin); the attenuated strain is named C. novyi-NT. The spores of C. novyi-NT germinate in hypoxic regions of tumor tissue and destroy tumor cells by secreting lipases, proteases, and degradative enzymes.[10c] The efficacy and relative safety of this approach have recently been demonstrated in companion canines with naturally occurring sarcomas; human clinical trials are now ongoing in patients with advanced solid tumors (NCT03435952).[10d, 11] Recently, our group reported a computed tomography (CT) image-guided cancer treatment using C. novyi-NT spores coated with branched gold nanoparticles.[12] Although the nanoparticle-coated spores were successfully delivered to hypoxic cancer tissues via CT imaging, and effectively treated central tumor regions by locally germinated C. novyi-NT, cancer cells at the normoxic peripheral rim remained alive resulting in a potential tumor recurrence. To prevent cancer recurrence and metastasis in both PDT or bacteriolytic therapy, a new combination therapy is necessary to treat whole tumor including the rim and central region effectively.
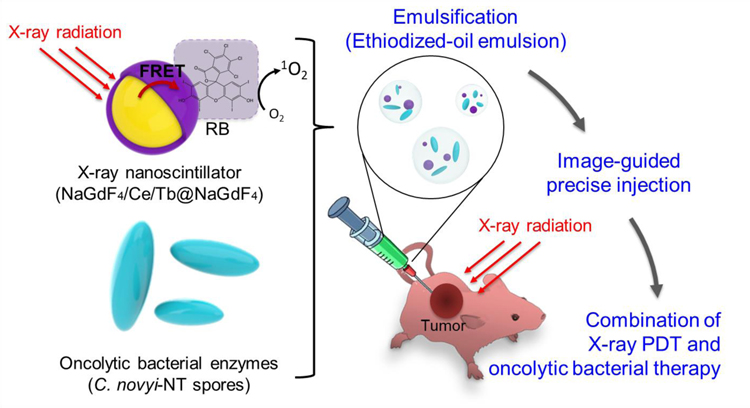
We hypothesized that a nano-bio therapeutic emulsion formulated with multi-functional nanoscintillators and C. novyi-NT spores would allow synergistic image-guided combinational cancer therapy treating both normoxic peripheral tumor and central hypoxic tumor. To test this hypothesis, PS coated core/shell-structured lanthanide-doped nanoscintillators (PS-NSs) that are visible in MRI and generate ROS through fluorescence resonance energy transfer (FRET) upon X-ray irradiation were synthesized, and oncolytic C. novyi-NT bacteria spores were combined for enhancing therapeutic efficacy (Scheme 1). In this novel form of combination therapy, a nano-bio combination of PS-NSs and C. novyi-NT spores were emulsified using an ethiodized oil (EO), which is frequently used in the clinic for interventional CT image-guided cancer therapy. We tested the therapeutic potential of this nano-bio therapeutic emulsion in a human prostate tumor-bearing mouse model. Intra-tumoral injection was performed with CT-image guidance (Scheme 1). Since both injected EO and PS-NSs have CT imaging capability, it is difficult to confirm the in vivo distribution of the PS-NSs through CT imaging. Thus, the intra-tumoral distribution of Gd-containing PS-NSs was monitored by T1-weighted MRI. Finally, we confirmed the therapeutic efficacy of the combination treatment using the nano-bio therapeutic emulsion by immunohistochemical analysis.
Scheme 1.
Schematic illustration of nano-bio therapeutic emulsion formulated with nanoscintillators, anaerobic C. novyi-NT spores, and ethiodized oil for the synergistic combination of X-ray photodynamic and anaerobic bacteriolytic therapy. In this system, RB-NSs that generate reactive oxygen species (ROS) through fluorescence resonance energy transfer (FRET) upon X-ray irradiation and oncolytic anaerobic bacterial spores (C. novyi-NT spores) form a nano-bio therapeutic emulsion with ethiodized oil (EO). Image-guided infused nano-bio therapeutic emulsion allowing a synergistic combination of X-ray PDT (ROS) and anaerobic bacterial cancer therapy effectively treats both normoxic peripheral tumor and central hypoxic tumor,
2. Results and Discussion
2.1. Photosensitizer-loaded lanthanide nanoscintillators
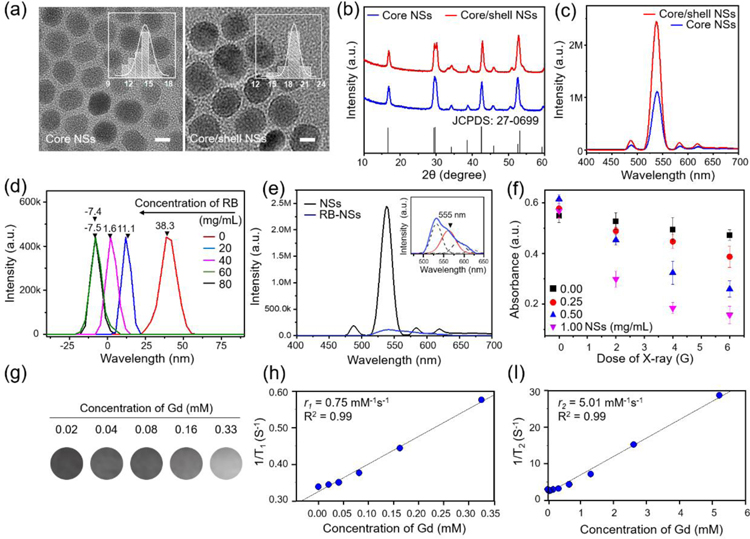
We synthesized monodispersed NaGdF4:15%Tb,10%Ce@NaGdF4 core/shell nanoparticles as nanoscintillators (NSs) using a thermal decomposition method.[13] Figure 1a shows the TEM images of the core and core/shell NSs. It can be clearly seen that the NSs are monodisperse and uniform with a spherical shape. The average sizes of the core and core/shell NSs are determined to be 14.5 ± 3.0 nm and 19.0 ± 1.8 nm, respectively, indicating the formation of a thin shell with a thickness of approx. 2.25 nm. As shown in Figure 1b, X-ray diffraction (XRD) patterns of the NSs agreed with the pattern of hexagonal β-NaGdF4 (JCPDS: 27–0699).[14] The Gd-based core was selected as a substrate to achieve epitaxial growth of shell containing Gd ions (a T1-weighted MRI contrast agent) and as an intermediate sublattice for energy transfer from sensitizer Ce3+ ions to activator Tb3+ ions which makes it possible to convert UV or X-ray radiation into visible light efficiently. Figure 1c shows fluorescence emission spectra of core and core/shell NSs (Figure 1b and S2, Supporting Information). Strong emission peaks were observed at 493, 544, 588, and 622 nm, corresponding to the characteristic bands of the activator Tb3+ under excitation with UV light; the bands are due to electronic transitions from the excited 5D4 state to the 7FJ (J=6–3) ground states.[15] Note that Ce3+ ion-doped nanostructures show similar fluorescence patterns when using X-ray radiation instead of UV light because of the photocatalytic property of Ce3+ ions.[16] We also confirmed X-ray induced fluorescence emission of NSs during the irradiation X-ray beam (Figure S1, Supporting Information). Rose Bengal (RB), an anionic water-soluble xanthene dye, was used as a PS for FRET from the NS donor due to the exact spectral match between the emission of the NS and the absorption of RB (Figure S2, Supporting Information). To promote electrostatic interaction between NS and RB, the hydrophobic surfaces of pre-synthesized NSs were exchanged for amine functionality that has positively charged hydrophilic surfaces via a ligand exchange and ionization process using dopamine. The mean hydrodynamic diameter of the water-soluble NS was 40.03 nm, and zeta-potential was +38.3 mV (Figure S3, Supporting Information). The zeta-potentials of the NSs mixed with RB at various concentrations in deionized water are shown in Figure 1d. Zeta-potentials of the colloidal solutions decreased as the concentration of RB increased; at 60 µg/mL RB, zeta-potential was at its minimum value, −7.5 mV. Using fluorescence spectra and luminescence lifetime decay curves, we investigated energy transfer from NS to RB in RB-loaded NSs (RB-NSs) solution. Figure 1e shows that the green emission bands of NSs were drastically quenched after RB loading, whereas a new emission at 555 nm appeared, corresponding to the characteristic bands of RB (Figure S5, Supporting Information). Further energy transfer analysis was carried out by measuring luminescence decay at 544 nm from NSs and RB-NSs. The luminescence decay curves were fitted to a mono-exponential function to obtain the luminescence lifetime (Figure S4, Supporting Information). The luminescence lifetime (2.225 ms) of RB-NSs at 544 nm was shorter than that of NSs (3.626 ms), indicating the occurrence of energy transfer from NSs to RB.[17] To confirm the ROS generation capability of RB-NSs by X-ray irradiation, we quantified ROS by using 1,3-diphenylisobenzofuran (DPBF) at various X-ray doses and RB-NS concentrations; when ROS are generated, the absorbance of DPBF decreases.[18] As shown in Figure 1f, the absorbance of DPBF decreased as X-ray intensity increased. In addition, the production of ROS increased with RB-NSs concentration. This result indicated that the NSs could absorb X-rays and effectively activate the PS (i.e., RB) on the surface, resulting in the production of ROS. Although X-ray irradiation without RB-NSs showed a slight signal reduction, it was shallow compared to the groups containing RB-NSs. Taken together, these results indicate that RB was successfully loaded onto the surface of NSs and that RB-NSs could produce ROS upon irradiation with X-rays. To study the MR imaging capability of the NSs, we performed an MR phantom study at various NS concentrations using 7T MRI (Figure 1g–i). Gd-free shell NSs yielded no T1 MR signal (data not shown), whereas Gd-doped NSs yielded imaging signals that increased with NS concentration. The r1 and r2 values were 0.75 and 5.01 mM−1s−1, respectively. The r2/r1 value was 6.68, which was higher than other Gd-based T1 contrast agents (e.g., Gd-DTPA (Magnevist) at 7T MR scanner).[19] The relatively high r2/r1 ratio of RB-NS is thought to be due to the negative lattice shielding effect with increasing Gd-free shell thickness.[20] But our RB-NS shows a lower r2/r1 level compared to the exceedingly small iron oxide nanoparticles developed as T1 MRI contrast agents.[19] Thus the RB-NSs are suitable therapeutic agents for X-ray PDT, as well as for T1 MR imaging for image-guided therapy.
Figure 1.
Characterizations of lanthanide-doped nanoscintillators (NSs) and Rose Bengal (RB)-loaded NSs (RB-NSs). (a) TEM images of NaGdF4:Tb,Ce (core NSs) and NaGdF4:Tb,Ce@NaGdF4 (core/shell NSs). The insets show size distribution. Scale bar, 10 nm. (b) X-ray diffraction (XRD) patterns of core and core/shell NSs. (c) Room-temperature emission spectra of core and core/shell NSs under excitation at 254 nm. (d) Zeta-potentials of water-soluble NSs with different concentrations of RB. (e) Emission spectra of NS and RB-NSs under excitation at 254 nm. Inset: Emission spectrum of NSs (black) and RB (red), respectively. (f) Reactive oxygen generation (ROS) of RB-NSs under a single fraction X-ray irradiation (n=3, independent experiments). (g–i) (g) T1-weighted MR image and (h) r1 (1/T1) and (i) r2 (1/T2) relaxivity of RB-NSs as a function of Gd concentration from RB-NSs, measured using 7T MR scanner. Scale bar: 10 nm.
2.2. In vitro cytotoxicity of RB-NS upon X-ray irradiation
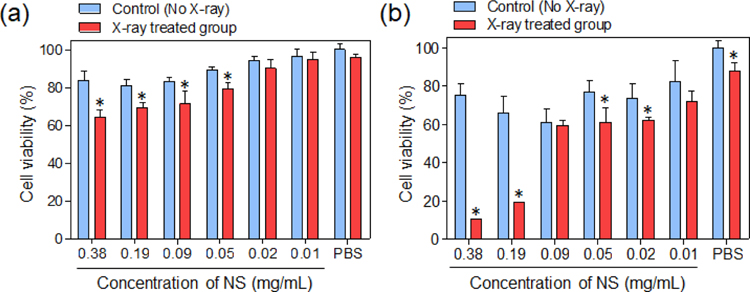
Next, we investigated whether cancer cells were killed by the RB-NS–generated ROS following X-ray irradiation in vitro (Figure 2). Since C. novyi-NT is an anaerobic bacterium, co-culture with mammalian cancer cells is impossible. Thus, the study did not perform in vitro testing with C. novyi-NT. Prostate cancer is a malignant tumor that starts around the prostate gland and can spread to other major organs.[21] Because image-guided local treatments are often used to treat prostate tumors in the clinic, we chose prostate cancer as the target disease for this study. Accordingly, we used PC3, human prostate cancer cells, for in vitro/in vivo experiments. PC3 cells were treated with various concentrations of RB-NSs and then irradiated with a single fraction of 6 Gy X-rays. The viability of cancer cells was measured at 24 and 72 h after X-ray treatment. At 24 h, the group treated with both RB-NS and X-rays exhibited slight toxicity relative to the no–X-ray control group (no X-ray) (Figure 2a). However, at 72 h, the group treated with RB-NS and X-ray exhibited 40% poorer survival than the control group. In the group treated X-ray irradiation only without RB-NSs, slight toxicity was observed at 72 h (Figure 2b). These results indicate that the ROS generated from RB-NS upon X-ray irradiation can effectively kill cancer cells. Although this study did not conduct studies on the toxicity caused by X-ray PDT in normal cells, previous studies showed a significant improvement in the therapeutic effect at the site where radiosensitizers were injected.[22]
Figure 2.
In vitro cytotoxicity of RB-NS–treated PC3 cells, with or without X-ray irradiation. Viability was measured (a) 24 h and (b) 72 h after X-ray irradiation of PC3 cells treated with RB-NSs (n=4, *P<0.05).
2.3. In vivo image-guided delivery of nano-bio therapeutic emulsion to tumor
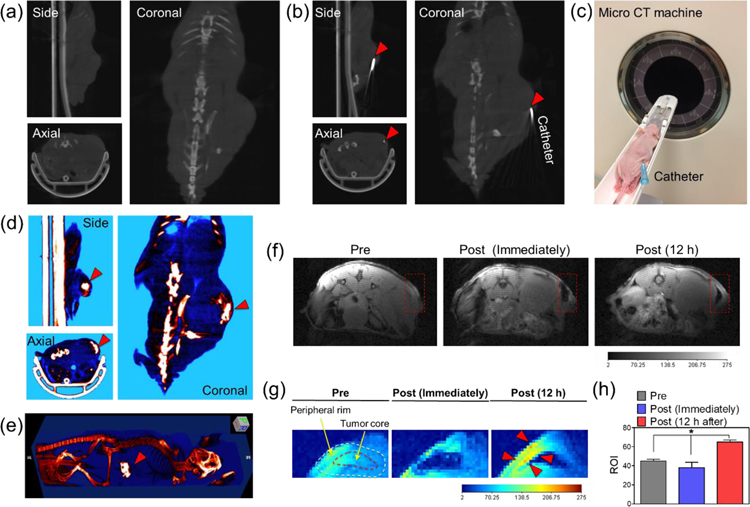
To perform combination X-ray PDT and oncolytic bacterial treatment with image guidance, we prepared a nano-bio therapeutic emulsion formulated with synthesized RB-NS, C. novyi-NT spores, and EO. The nano-bio therapeutic emulsion was prepared by mixing the aqueous solution containing RB-NS and bacterial spores (BSs) with EO at a ratio of 1:1.5 (v/v), as described previously.[23] In clinical interventional oncology, the formation of an emulsion by mixing anti-cancer agents with EO is a commonly used procedure for chemoembolization.[24] Typically, the EO emulsion sizes range from tens to hundreds of micrometers.[25] Also, EO can be used as a CT contrast agent because it contains iodine, which absorbs X-rays. To inject the nano-bio therapeutic emulsion into the tumor with CT-image guidance, we placed a catheter at the center of the tumor. As shown in Figure 3a–c, the catheter was successfully positioned at the center of the tumor. CT images clearly showed that the injected emulsion accumulated in the tumor site (Figure 3d and e). In addition, we monitored the distribution of RB-NSs in the tumor over time by MR imaging (Figure 3f–h). Immediately after injection, the T1 signal tended to decrease due to signal attenuation by the injected lipid emulsion. However, the T1 signal was significantly increased around the peripheral rim of the tumor 12 h after injection (Figure 3g and h), implying that the released RB-NS from the emulsion could migrate around the normoxic peripheral rim of the tumor over time. Taken together, these results indicate that NPs and BSs can be efficiently delivered to the tumor site through image guidance and that the distribution of the RB-NSs in the tumor can also be confirmed using imaging equipment.
Figure 3.
In vivo image-guided injection of the nano-bio therapeutic emulsion into PC3 tumor-bearing mice. (a–b) Micro CT image of the animal model (a) before and (b) after insertion of the catheter in the core region of the tumor. (c) Digital photograph image of the animal model, with a catheter inserted into the tumor site for micro CT imaging. (d) Micro CT and (e) 3D reconstructed the micro CT image of the animal model after the infusion of the nano-bio therapeutic emulsion into the tumor site. (f–g) T1-weighted MR and color-coded MR image of the animal model before and after injection of the nano-bio therapeutic emulsion. (h) Changes in ROI of the tumor site over time in T1-weighted MR images (n=3, *P<0.002).
2.4. In vivo antitumor efficacy of nano-bio therapeutic emulsion upon X-ray irradiation
To evaluate the combined therapeutic effect of nano-bio emulsion, we injected the emulsion into the tumor under CT-image guidance, and then performed X-ray irradiation. Based on the MR images in Figure 3f–h, X-ray irradiation was performed at 12 h after injection to allow NPs to diffuse into the peripheral rim region of the tumor thoroughly. Besides, the X-ray irradiation can damage the bacterial genes and thereby affect their growth, which is why the X-ray irradiation was performed 12 h after the injection. In future studies, the effects of X-ray radiation intensity and time on bacteria need to be investigated in more detail.
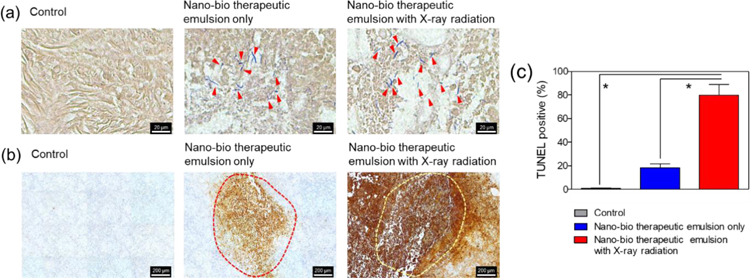
Generally, oxygen-rich peripheral rim sites are advantageous for the efficient production of cancer-killing ROS by X-ray PDT.[26] As shown in Figure 4a, bacteria germinated at the hypoxic tumor core injected with the emulsion (Gram staining, red arrows). The control means a group injected with PBS. Taken together, the imaging results in Figure 3 show that bacterial spores were germinated at the hypoxic tumor core, and the RB-NSs were released from the emulsion and diffuse into the normoxic peripheral tumor rim after the image-guided injection. The area of TUNEL-positive signal, which indicates cancer cells killed by apoptosis,[12, 27] was more significant in the group treated with emulsion and X-ray than in the group injected with emulsion alone (Figure 4b). These data are quantified in Figure 4c, revealing a statistically significant difference. In a previous study by our group,[12] C. novyi-NT spores killed only cancer cells in the hypoxic tumor core region; the cancer cells in the normoxic peripheral tumor rim site remained alive. By contrast, in this study, the combination of X-ray PDT with NSs and bacterial treatment using the nano-bio therapeutic emulsion effectively treated both the hypoxic central tumor region and normoxic peripheral tumor rim. Thus, the nano-bio therapeutic emulsion allowing combined use of X-ray PDT with bacterial therapeutics may not only improve therapeutic efficacy, but also decrease tumor recurrence relative to conventional monotherapies. This study is a proof of concept study of the combination of bacteriolytic therapy with X-ray PDT. Future work will be conducted in more detail about the antitumor effects with various imaging modalities.
Figure 4.
In vivo therapeutic efficacy of combination X-ray photodynamic therapy (PDT) and C. novyi-NT bacterial therapy using the nano-bio therapeutic emulsion in the PC3 mouse tumor model. (a) Gram and (b) TUNEL staining of tumor tissue subjected to the indicated treatments: control, nano-bio therapeutic emulsion only, and nano-bio therapeutic emulsion plus X-ray radiation. The inner part of the red and yellow dotted line represents the hypoxic tumor core. (c) Quantitative analysis of TUNEL staining results. The control means a group injected with PBS (n=4, *P<0.002).
3. Conclusion
In this study, we formulated a nano-bio therapeutic emulsion that can perform synergistic combination X-ray PDT and bacterial cancer therapy. The multifunctional PS-NSs were successfully synthesized with a core/shell structure that enabled T1 MRI imaging and killing of cancer cells by conversion of X-rays into light energy, thereby activating PSs on the NS and generating ROS. PS-NSs and C. novyi-NT BSs were readily combined into EO for a nano-bio therapeutic emulsion. The formulated emulsion was successfully injected into tumor sites under CT-image guidance. The distribution of PS-NS in the tumor was confirmed by MR imaging. Application of X-ray to tumors injected with the nano-bio therapeutic emulsion yielded a robust antitumor effect treating both central and peripheral tumors. We believe that the nano-bio therapeutic emulsion for a synergistic image-guided combination therapeutic strategy has the potential to treat various solid tumors effectively. Further, these nano-bio therapeutic emulsion and combination therapy, together with recently characterized immune-responses of local therapies, can open new effective immune-therapeutic options.
4. Materials and methods
4.1. Materials
Yttrium (III) acetate hydrate, cerium (III) acetate hydrate, terbium (III) acetate hydrate, gadolinium (III) acetate hydrate (99.9%), oleic acid (OA, 90%), 1-octadecene (ODE, 90%), sodium hydroxide (NaOH), ammonium fluoride (NH4F), ethanol (absolute), methanol, cyclohexane, tetrahydrofuran (THF), dopamine hydrochloride, hydrochloric acid (HCl), peptone, L-cysteine, maltose, sodium phosphate dibasic (Na2HPO4), and tetrahydrofuran (THF, ≥97%) were purchased from Sigma-Aldrich (St Louis, Mo, USA). C. novyi-NT spores were kindly provided by BioMed Valley Discoveries (Kansas City, MO, USA). Lipiodol™ was obtained from Guerbet (Villepinte, France).
4.2. Synthesis of Rose Bengal-loaded nanoscintillators (RB-NS)
NaGdF4:Tb,Ce (core nanoscintillators, core NSs) were synthesized by a general thermal decomposition strategy using lanthanide acetates as precursors.[13a] An aqueous solution (1 mL) of Ln(CH3CO2)3 (Ln=0.3 mmol Gd, 0.06 mmol Tb and 0.04 mmol Ce, total 0.4 mmol) was added to a three-neck round flask containing OA (3 mL) and ODE (7 mL). The mixture was heated at 150°C for 30 min to form lanthanide-oleate complexes and then cooled down to 50°C. Subsequently, a methanol solution (5 mL) containing NaOH (1 mmol) and NH4F (1.6 mmol) was added, and the resultant solution was stirred for 30 min at 50°C. The solution was heated to 100°C to evaporate the remaining methanol. After that, the solution was heated to 290°C under argon for 1.5 h and then cooled down to room temperature. The core NSs were precipitated by adding ethanol to the flask. The precipitated NSs were collected by centrifugation and washed several times with ethanol and methanol. The core NSs were re-dispersed in cyclohexane. NaGdF4:Tb,Ce@NaGdF4 (core/shell NSs) were synthesized by a similar procedure as a synthesis of core NSs. An aqueous solution (1 mL) containing Gd(CH3CO2)3 (total 0.4 mmol) was added to a round flask containing OA (3 mL) and ODE (7 mL). The mixture was heated at 150°C for 30 min and then cooled down to 50°C. Subsequently, the core NSs and methanol solution (5 mL) containing NaOH (1 mmol) and NH4F (1.6 mmol) were added, and the resultant solution was stirred for 30 min at 50°C. The solution was heated to 290°C under argon for 1.5 h and then cooled down to room temperature. The NSs were collected and washed several times with ethanol and methanol. The core/shell NSs were re-dispersed in THF. The Gd-free NSs were synthesized by replacing Gd with Y based on the above synthesis procedure. For the ligand exchange of the NSs, the NSs in THF were mixed with dopamine hydrochloride in water.[22b] After incubation for 5 h at 50°C, the solution was cooled down to room temperature. After that, HCl was added into the flask and washed twice with water. The positive charged NSs were mixed with RB for 30 min. The resultant solution was centrifuged and washed twice with water. Transmission electron microscopy (TEM) using a Talos F200X instrument (FEI Co., USA) was used to characterize the morphologies of NSs at an accelerating voltage of 200 kV. A Zetasizer Nano ZS instrument (Malvern Co., UK) was used to determine zeta potentials of NSs. The XRD patterns of NSs were characterized by an XRD-7000 diffractometer. Fluorescence spectra were recorded using Shimadzu RF-6000 spectrofluorophotometer (Shimadzu, Japan). Lifetime decay curves were fitted with a single exponential decay law.
4.3. Reactive oxygen species (ROS) detection
Following our previous report,[22b] 1,3-diphenylisobenzofuran (DPBF) was used to confirm the ROS generation of nanoscintillators under X-ray. 1 mM DPBF was dissolved in 1 mL of ethanol and mixed with 1 mL of the aqueous solution of different concentrations of nanoparticles. The mixture of DPBF and nanoparticles was irradiated with different intensity X-rays using an X-ray irradiator (RS-2000, RadSource Technologies, Inc., Suwanee, GA, USA). Radiation intensity was 0–6 Gy, and the irradiation intensity was controlled by adjusting the X-ray exposure time. The change of absorbance of DPBF (at 412 nm) in solution after X-ray irradiation was measured by a UV/Vis spectrometer (Cytation3, BioTek, USA).
4.4. MRI property characterization of RB-NS
Phantoms were prepared with various NP concentrations of RB-NS. The RB-NS were suspended in 1% agar phantoms and pipetted into microtubes. T1 relaxation times of RB-NS was determined using a 7T MR scanner (BioSpec, Bruker, Billerica, MA, USA). T1 was measured using a gradient-echo sequence with different flip angles (FA=5 – 30°) in the same TR = 36 ms and TE = 2.64 ms. T1 measurements were performed using a nonlinear fit to change in the mean signal intensity of each sample as a function of FA. The r1 and r2 relaxivity values were determined through the curve fitting of relaxation rate (s−1) versus the Gd concentration (mM) (SigmaPlot v.10.0, Systat Software Inc., San Jose, CA, USA). The amount of Gd contained in the RB-NS was measured using inductively coupled plasma optical emission spectrometry (ICP-OES, Perkin-Elmer Model Optima 4300 DV, Palo Alto, CA, USA) after digestion in 70% nitric acid.
4.5. Culture and purification of C. novyi-NT spores
C. novyi-NT spores were cultured and purified according to the previously reported method.[10a, 12] C. novyi-NT spores were cultured in the sporulation medium for 2 weeks under an anaerobic environment at 37 °C. The sporulation medium contained peptone (30 g), L-cysteine (0.5 g), maltose (10 g), Na2HPO4 (5 g), and 5% wt/vol of dried cooked meat particles (Difco Laboratories, Detroit, Mich, USA) per 1 L of medium. The mature spores were purified using a Percoll (GE Healthcare, Amersham Place, UK) density gradients column (55 and 75%). The purified spores were washed 5 times with PBS.
4.6. In vitro cell culture
The human PC3 prostate cancer cells (PC3, ATCC, Manassas, VA, USA) were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium with 10% fetal bovine serum (FBS) and supplemented with 1% penicillin at 37 °C, under 5% CO2, as previously reported.[28]
In vitro cytotoxicity test:
PC3 cells with the concentration of 1 × 104 cells were plated in each well of a 96-well plate and cultured for 12 h. The different concentrations of RB-NS with a range of 0–38 mg/mL were treated onto the cells and incubated for 12 h. For the treatment of X-ray radiation, the well-plate was placed onto the center of the shelf in an X-ray irradiator and was exposed upon a radiation dose of 6 Gy. The cell viability was measured using a colorimetric Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kyoto, Japan) after 24 or 48 h after the X-ray irradiation. The cell experiments were performed four times independently and analyzed statistically.
4.7. In vivo mouse xenograft tumor model
Animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Northwestern University. To generate tumor model, five million PC3 cells (human prostate cancer cell line) were subcutaneously injected into the right flank of BALB/c nude mice (female, 4-weeks old, from Charles River, Wilmington, MA, USA). Tumors were allowed to grow for 1–2 weeks after xenograft prior to imaging and spore injection procedures.
4.8. Preparation of nano-bio therapeutic emulsion
RB-NS and BS were dispersed in 50 µL of PBS solution. The mixture of RB-NS (1 mg) and BS (3 × 108 C. novyi-NT spores) was mixed with 75 μL of EO (i.e., Lipiodol™) and then subjected to vortex for 1 min to obtain the CT visible emulsion.
4.9. In vivo image-guided combination tumor treatment using nano-bio therapeutic emulsion
The tumor-bearing mice received a CT-guided intra-tumoral injection of 125 µL of the prepared RB-NS/BS/EO emulsion by using a 23G needle (BD Biosciences, San Jose, CA, USA). As a control group, PBS was injected through intra-tumoral injection. To prevent the destabilization of emulsion, the sample solutions were gently vortexed for 20 seconds before the injection. X-ray irradiation was performed at 12 h after injection to induce bacterial germination. For the X-ray irradiation, mice were anesthetized with ketamine and xylazine and irradiated with a single dose of radiation (6 Gy) on the tumor region with shielding the rest of the body using a lead shield (Braintree Scientific, MA, USA). Then, the mice were euthanized in 48 h after treatment, and the tumor tissues were collected for histological analysis.
4.10. In vivo X-ray CT imaging
X-ray CT studies were performed using a micro-CT imaging system (NanoPET/CT®, Mediso Ltd-Bioscan Inc., Hungary-US) at the Center for Translational Imaging at NU. For in vivo CT imaging, PC3 tumor xenografted mice were imaged with an applied X-ray tube voltage of 35 keV. 3D-rendered images were generated using OsiriX software (OsiriX Foundation, Geneva, Switzerland, version 3.7.1). Each sample was injected into three mouse tumor models and then imaged independently. These results were statistically analyzed using GraphPad Prism software (GraphPad Prism Software Inc., San Diego, CA, USA).
4.11. In vivo MR imaging
MR scans were performed in both coronal and transversal orientations using a spin-echo sequence with following parameters: TR/TE=500/11.10 ms, flip angle 180°, 1 mm slice thickness, and respiratory triggering with MRI-compatible small animal gating system (Model 1025, SA Instruments, Stony Brook, NY, USA). The change of the T1 signal (region of interest, ROI) in the tumor was quantitatively analyzed using JIM 6.0 (Xinapse Systems, http://www.xinapse.com). Each sample was injected into three mouse tumor models and then imaged independently. These results were statistically analyzed using GraphPad Prism software (GraphPad Prism Software Inc., San Diego, CA, USA).
4.12. Histology and immunohistochemistry analysis
The harvested tumor tissues were fixed with 10% neutral formalin 4 solution and then submitted to Mouse Histology and Phenotyping Laboratory (MHPL, Northwestern University, USA) core for Gram-positive, H&E and TUNEL staining with 5 μm slice thickness. All slides were analyzed using a TissueFAXS microscope (TissueGnostics GmbH, Vienna, Austria). The quantitative analysis of TUNEL-positive cells in the tissues was performed using Image J software (National Institutes of Health, Bethesda, MD, USA).
4.13. Statistical analysis
Statistical significance of differences between two groups was determined using Student’s unpaired t-test. P<0.05 was considered to indicate a significant difference. All statistical analyses were performed using GraphPad Prism software (GraphPad Prism Software Inc., San Diego, CA, USA).
Supplementary Material
Acknowledgments
This work was supported by grants R01CA218659 and R01EB026207 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering and a grant from the Korea Institute of Science and Technology Institutional Program (2E29290/CAP-16–02-KIST), and the Basic Science Research Program (2018R1C1B6001120) through an NRF grant funded by the Korean government (MSIT).
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library.
References
- [1].a) Park W, Park SJ, Cho S, Shin H, Jung YS, Lee B, Na K, Kim DH, J Am Chem Soc 2016, 138, 10734; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim DH, Guo Y, Zhang ZL, Procissi D, Nicolai J, Omary RA, Larson AC, Adv Healthc Mater 2014, 3, 714; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Li FY, Du Y, Liu JA, Sun H, Wang J, Li RQ, Kim D, Hyeon T, Ling DS, Adv Mater 2018, 30. [DOI] [PubMed]
- [2].a) Luo DD, Carter KA, Miranda D, Lovell JF, Adv Sci 2017, 4; [DOI] [PMC free article] [PubMed]; b) Wang B, Lin WM, Mao ZW, Gao CY, J Mater Chem B 2018, 6, 3145. [DOI] [PubMed] [Google Scholar]
- [3].a) Fang-Yen C, Gabel CV, Samuel AD, Bargmann CI, Avery L, Methods Cell Biol 2012, 107, 177; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Foster TH, Giesselman BR, Hu R, Kenney ME, Mitra S, Transl Oncol 2010, 3, 135; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Dolmans DE, Fukumura D, Jain RK, Nat Rev Cancer 2003, 3, 380; [DOI] [PubMed] [Google Scholar]; d) Castano AP, Mroz P, Hamblin MR, Nat Rev Cancer 2006, 6, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Henderson TA, Neural Regen Res 2016, 11, 563; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liang L, Xie X, Loong DTB, All AH, Huang L, Liu X, Chem Eur J 2016, 22, 10801; [DOI] [PubMed] [Google Scholar]; c) Shao W, Chen G, Kuzmin A, Kutscher HL, Pliss A, Ohulchanskyy TY, Prasad PN, J Am Chem Soc 2016, 138, 16192; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Park W, Cho S, Han J, Shin H, Na K, Lee B, Kim D-H, Biomater Sci 2018, 6, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Khdair A, Chen D, Patil Y, Ma L, Dou QP, Shekhar MP, Panyam J, J Control Release 2010, 141, 137; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) He C, Liu D, Lin W, ACS nano 2015, 9, 991; [DOI] [PubMed] [Google Scholar]; c) Park H, Park W, Na K, Biomaterials 2014, 35, 7963. [DOI] [PubMed] [Google Scholar]
- [6].a) Abbas M, Zou Q, Li S, Yan X, Adv Mater 2017, 29, 1605021; [DOI] [PubMed] [Google Scholar]; b) Vijayaraghavan P, Liu CH, Vankayala R, Chiang CS, Hwang KC, Adv Mater 2014, 26, 6689; [DOI] [PubMed] [Google Scholar]; c) Han J, Park W, Park S.-j., Na K, ACS Appl Mater Inter 2016, 8, 7739. [DOI] [PubMed] [Google Scholar]
- [7].a) Zhang C, Zhao K, Bu W, Ni D, Liu Y, Feng J, Shi J, Angew Chem Int Ed 2015, 54, 1770; [DOI] [PubMed] [Google Scholar]; b) Wang GD, Nguyen HT, Chen H, Cox PB, Wang L, Nagata K, Hao Z, Wang A, Li Z, Xie J, Theranostics 2016, 6, 2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gil M, Bieniasz M, Seshadri M, Fisher D, Ciesielski M, Chen Y, Pandey R, Kozbor D, Br J Cancer 2011, 105, 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR, Lin W, J Am Chem Soc 2016, 138, 12502; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, Dong Z, Zhu W, Peng R, Liu Z, ACS Nano 2017, 11, 4463. [DOI] [PubMed] [Google Scholar]
- [10].a) Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B, Proc Natl Acad Sci USA 2001, 98, 15155; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Agrawal N, Bettegowda C, Cheong I, Geschwind J-F, Drake CG, Hipkiss EL, Tatsumi M, Dang LH, Diaz LA, Pomper M, Proc Natl Acad Sci USA 2004, 101, 15172; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bettegowda C, Huang X, Lin J, Cheong I, Kohli M, Szabo SA, Zhang X, Diaz LA Jr, Velculescu VE, Parmigiani G, Nature biotechnology 2006, 24, 1573; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Staedtke V, Roberts NJ, Bai R-Y, Zhou S, Genes Dis 2016, 3, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Theys J, Lambin P, Ann Transl Med 2015, 3 S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park W, Cho S, Huang X, Larson AC, Kim DH, Small 2017, 13, 1602722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Lee J, Gordon AC, Kim H, Park W, Cho S, Lee B, Larson AC, Rozhkova EA, Kim D-H, Biomaterials 2016, 109, 69; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mi C, Gao H, Li F, Xu S, Colloid Surface A 2012, 395, 152. [Google Scholar]
- [14].Ou X-Y, Guo T, Song L, Liang H-Y, Zhang Q-Z, Liao J-Q, Li J-Y, Li J, Yang H-H, Anal Chem 2018, 90, 6992. [DOI] [PubMed] [Google Scholar]
- [15].Elmenoufy AH, Tang Y. a., Hu J, Xu H, Yang X, Chem Comm 2015, 51, 12247. [DOI] [PubMed] [Google Scholar]
- [16].Zhong X, Wang X, Zhan G, Tang Y. a., Yao Y, Dong Z, Hou L, Zhao H, Zeng S, Hu J, Cheng L, Yang X, Nano Lett 2019, 19, 8234. [DOI] [PubMed] [Google Scholar]
- [17].Kuk S, Lee BI, Lee JS, Park CB, Small 2017, 13, 1603139. [DOI] [PubMed] [Google Scholar]
- [18].a) Gomes A, Fernandes E, Lima JL, J Biochem Biophys Methods 2005, 65, 45; [DOI] [PubMed] [Google Scholar]; b) Ohyashiki T, Nunomura M, Katoh T, BBA-Biomembranes 1999, 1421, 131. [DOI] [PubMed] [Google Scholar]
- [19].Wei H, Bruns OT, Kaul MG, Hansen EC, Barch M, Wiśniowska A, Chen O, Chen Y, Li N, Okada S, Proc Natl Acad Sci USA 2017, 114, 2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee SH, Kim BH, Na HB, Hyeon T, Wiley Interdiscip Rev: Nanomed Nanobiotechnol 2014, 6, 196. [DOI] [PubMed] [Google Scholar]
- [21].Dall’Era MA, Cooperberg MR, Chan JM, Davies BJ, Albertsen PC, Klotz LH, Warlick CA, Holmberg L, Bailey DE Jr, Wallace ME, Cancer-Am Cancer Soc 2008, 112, 1650. [DOI] [PubMed] [Google Scholar]
- [22].a) Bonvalot S, Le Pechoux C, De Baere T, Kantor G, Buy X, Stoeckle E, Terrier P, Sargos P, Coindre JM, Lassau N, Clin Cancer Res 2017, 23, 908; [DOI] [PubMed] [Google Scholar]; b) Cho S, Lee B, Park W, Huang X, Kim D-H, ACS Appl Mater Inter 2018, 10, 27570; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Goswami N, Luo Z, Yuan X, Leong DT, Xie J, Mater Horiz 2017, 4, 817. [Google Scholar]
- [23].Jeon MJ, Gordon AC, Larson AC, Chung JW, Kim YI, Kim D-H, Biomaterials 2016, 88, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a) Mabed M, Esmaeel M, El-Khodary T, Awad M, Amer T, Eur J Cancer Care 2009, 18, 492; [DOI] [PubMed] [Google Scholar]; b) Ono Y, Yoshimasu T, Ashikaga R, Inoue M, Shindou H, Fuji K, Araki Y, Nishimura Y, Am J Clin Oncol 2000, 23, 564; [DOI] [PubMed] [Google Scholar]; c) Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, J Hepatol 2007, 46, 474. [DOI] [PubMed] [Google Scholar]
- [25].a) Ahnfelt E, Degerstedt O, Lilienberg E, Sjögren E, Hansson P, Lennernäs H, J Drug Deliv Sci Tec 2019, 53, 101143; [Google Scholar]; b) Tanaka T, Masada T, Nishiofuku H, Fukuoka Y, Sato T, Tatsumoto S, Marugami N, Higashi S, Kichikawa K, Eur Radiol 2018, 28, 2203. [DOI] [PubMed] [Google Scholar]
- [26].a) Busch TM, Wileyto EP, Emanuele MJ, Del Piero F, Marconato L, Glatstein E, Koch CJ, Cancer Res 2002, 62, 7273; [PubMed] [Google Scholar]; b) Zhou Z, Song J, Nie L, Chen X, Chem Soc Rev 2016, 45, 6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Labat-Moleur F, Guillermet C, Lorimier P, Robert C, Lantuejoul S, Brambilla E, Negoescu A, J Histochem Cytochem 1998, 46, 327. [DOI] [PubMed] [Google Scholar]
- [28].a) Desai B, Rogers MJ, Chellaiah MA, Molecular cancer 2007, 6, 18; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gupta A, Cao W, Chellaiah MA, Mol Cancer 2012, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.