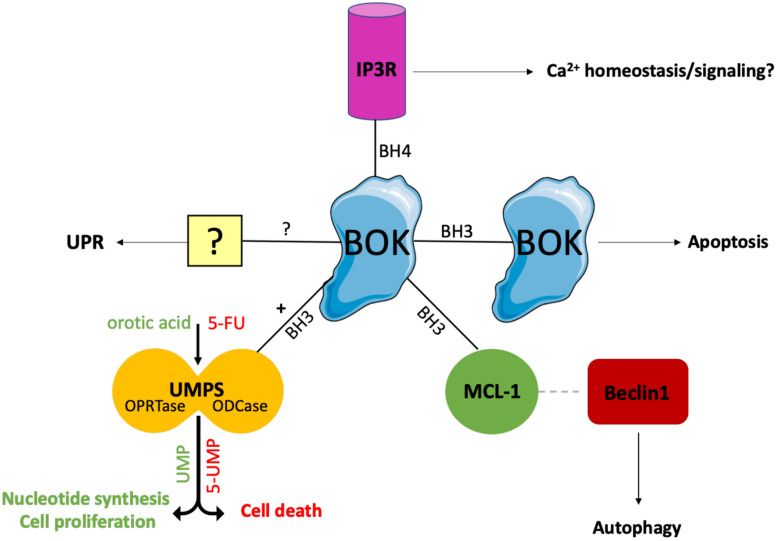
FIGURE 1.
Interaction partners of BOK. Only few BOK interacting partners have been described so far. Using yeast two-hybrid screens, BOK was found to interact with MCL-1. This interaction has been proposed to promote autophagy, as BOK binding to MCL-1 frees MCL-1–bound Beclin-1, which in turn initiates the autophagy machinery. Moreover, BOK is constitutively bound to IP3Rs (IP3R1 and IP3R2) at the ER via its BH4 domain. IP3Rs may act as a regulatory sink for BOK to keep the levels of unbound (“free”) BOK low. Furthermore, co-immunoprecipitation experiments indicated that BOK may interact with itself in a BH3 domain–dependent manner. Whether BOK truly forms dimers or homo-oligomers and whether this oligomerization induces MOMP need to be further investigated. Recently, BOK was found to interact with and increase the activity of the enzyme UMPS via its BH3 domain. UMPS catalyzes the last two steps of the de novo synthesis of UMP and is central for metabolizing the chemotherapeutic drug 5-FU into its active metabolites. As a result, cells lacking BOK have a defect in uridine metabolism, leading to decreased cellular proliferation compared to WT controls. On the other hand, Bok– /– cells are resistant toward 5-FU treatment. Losing BOK seems to confer a selective advantage to CRC cells, and consequently, BOK expression levels are suggested to be useful as a prognostic marker in CRC and as a predictive marker for the efficacy of 5-FU treatment in CRC. While there is increasing evidence for a UPR-modulating role for BOK, the exact mechanism and possible interaction partners for UPR modulation and the ER stress response are still unknown.