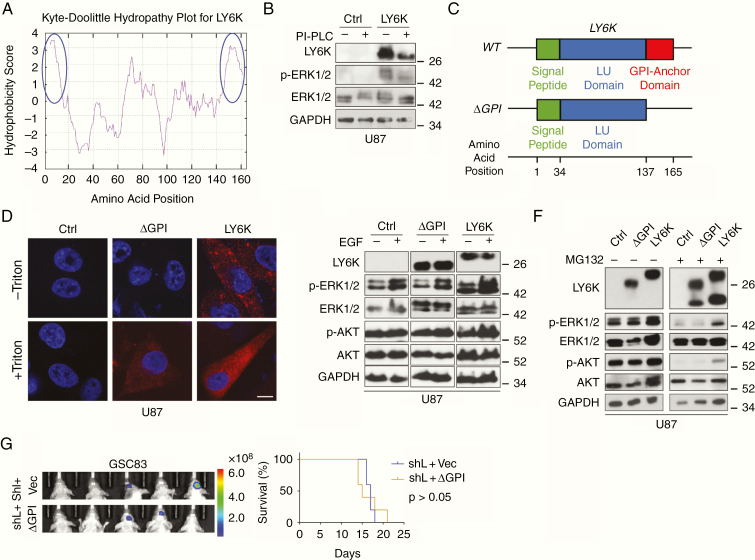
Fig. 5.
The GPI-anchor domain of LY6K is necessary for LY6K-enhanced ERK1/2 signaling and tumorigenicity. (A) Kyte–Doolittle Hydropathy Plot analysis. LY6K fits the typical hydrophobicity profile for GPI-anchored proteins (see blue circles marking strong hydrophobic regions at N- and C-termini). (B) IB. Treatment with PI-PLC decreased molecular weight of decreased levels LY6K and showed accompanying suppression of p-ERK1/2 levels. (C) Schematic of the three domains of the transcribed sequence for LY6K-WT (signal peptide, LU domain, and GPI-anchor domain) and the constructed mutant LY6K-ΔGPI, which lacks the GPI-anchor domain. The predicted amino acid position corresponding to each domain is depicted below. (D) Immunofluorescent staining. Unlike the LY6K-ΔGPI mutant, LY6K-WT is present on the cell membrane. LY6K-ΔGPI could not be visualized in the absence of membrane permeabilization (-Triton). (E) IB. Expression of LY6K-WT enhanced p-ERK1/2 levels, whereas LY6K-ΔGPI failed to do so, even in the presence of EGF. (F) IB. Proteasomal inhibitor MG132 reduced LY6K-enhanced p-ERK1/2. (G) GBM xenograft experiments. In GSC83 cells with knockdown of endogenous LY6K, subsequent expression of LY6K-ΔGPI mutant failed to restore tumorigenicity. Left, BLI images. Right, Kaplan-Meier survival analysis. For all IB, U87 cells with indicated modifications were used. p-AKT/AKT were used as nonspecific proteins and GAPDH was a loading control. Scale bar in D is 10 μm. Data in B and D-G are representative from two to three independent experiments with similar results.