Abstract
In this article, we examine a computational model to explore the prevalence of a viral infectious disease, namely hand-foot-mouth disease, which is more common in infants and children. The structure of this model consists of six sub-populations along with two delay parameters. Besides, by taking advantage of the Atangana–Baleanu fractional derivative, the ability of the model to justify different situations for the system has been improved. Discussions about the existence of the solution and its uniqueness are also included in the article. Subsequently, an effective numerical scheme has been employed to obtain several meaningful approximate solutions in various scenarios imposed on the problem. The sensitivity analysis of some existing parameters in the model has also been investigated through several numerical simulations. One of the advantages of the fractional derivative used in the model is the use of the concept of memory in maintaining the substantial properties of the understudied phenomena from the origin of time to the desired time. It seems that the tools used in this model are very powerful and can effectively simulate the expected theoretical conditions in the problem, and can also be recommended in modeling other computational models in infectious diseases.
Keywords: Mathematical modeling of infectious diseases, The Atangana–Baleanu fractional derivative, Approximate solutions, Predictor–corrector scheme, Fractional delay differential equations
Introduction
Infectious diseases are a branch of science that deals with the diagnosis and treatment of diseases caused by microorganisms. While many infectious diseases such as tuberculosis, plague, leprosy, smallpox, and the flu have existed throughout the history of the world, humans have been fighting microorganisms for centuries. Although some infectious diseases, such as smallpox, have been eradicated through vaccination, new infectious diseases have emerged, such as AIDS and COVID-19. With globalization, global warming, and increasing travel, whether newly emerging viral diseases or resistant bacterial infections, infectious diseases remain at the forefront.
The hand-foot-mouth disease (HFMD) is one of the most common infectious diseases that affect many people around the world. The disease is caused by some viruses like Coxsackievirus A16 and Enterovirus 71. The disease is more common in children under 5 years old. More precisely, the prevalence of the disease is much more common in children under three years of age. Besides, there have been numerous cases of the disease in adults around the world. In most cases, patients have mild symptoms for 7 to 10 days [1]. Some of these symptoms are fever and flu-like symptoms, mouth sores, and skin rash. So far, two main ways of transmitting this contagious disease have been identified: the first way is transmitted through contact with an infected person or contact with tools used by infected people. The disease can also be spread through coughing or sneezing in the air and transmitted through the respiratory transmission to another person.
The first cases of HFMD cases were clinically reported in Canada and New Zealand in 1957. The disease has been reported in many parts of the world, including some parts of Asia, Europe, and the United States. The disease first appeared in Shanghai, China, and spread rapidly to other parts of the country, including Beijing, Shandong, and Jiangxi provinces. According to our information, the epidemic of this disease occurs in cycles of two or three years. The best time to peak the spread of the disease is usually in the summer. Heat and humidity are known as two main factors that aggravate the spread of the disease. The first case of the disease was seen in Thailand in 2003. In a short time, signs of this contagious disease were visible in all cities and provinces of this country [32]. The first national study describing a large number of deaths caused in this country by the disease during an outbreak in 2011 was presented in [46]. HFMD has also been found in many Indian major states [38].
Unfortunately, no specific curative treatment has been found for this viral disease. However, several simple preventive measures such as avoiding direct contact with infected people, quarantining infected children at school, cleaning common utensils regularly, and disinfecting contaminated surfaces have been confirmed as the most effective ways to reduce transmission of the virus to other people in the community.
Due to the high prevalence of this disease in different parts of the world and the importance of identifying and controlling the factors affecting its prevalence, many extensive kinds of research have been conducted by researchers from various scientific aspects. In [26], a new method for the disease prediction using GeoDetector and a Long Short-Term Memory neural network (LSTM) has been proposed. In [40], the authors applied optimal control theory to the HFMD model, including the treatment and vaccination interventions. They presented some control strategies based on minimizing the cost of the intervention and minimizing the number of infected people. Very recently, a fractional-order model has been utilized to describe the transmission of HFMD in [39]. In this paper, the authors considered two major cases of constant and optimal control. In each case, the existence and uniqueness of positive solutions and the sufficient conditions for the existence and stability of equilibriums were investigated. It is important to mention that their study on the optimality control conditions is based on Pontryagin’s maximum principle. Another interesting study on the disease was conducted to investigate the role of air pollution in the prevalence and incidence of the disease in the warm and cold seasons in Wuhan, China [47]. In [42], the authors investigated some strategies to control the disease using a system of ordinary differential with two delay parameters. To estimate and better understand the transmissibility of HFMD, a susceptible–infected–recovered (SIR) model has been utilized in [26]. They claim that the main reason for this choice is that the incubation period of the disease is less than one week. In [13], another new SIR model has been developed to fit the surveillance data containing valuable information on the severity of HFMD in order to accurately estimate the basic reproductive number () of the disease. In [27], a simple SEIR model has been examined to investigate the dynamics of the disease among young children. A discrete SEIADR (susceptible–exposed–infectious–asymptomatic–dead–recovered) epidemic model has been also developed in [34]. To read more articles, please refer to [12, 16, 17, 28, 31, 33, 45, 50, 51].
Fractional differential calculus has been used in modeling many phenomena in everyday real-world applications [18, 20–23, 25]. Therefore, due to the importance and scope of applications, many efficient numerical methods specific to each of these types of fractional operators have also been introduced [3–8, 10, 11, 15, 19, 24, 30, 35, 37, 43]. However, in [41] the author has conducted a review to point some possible problems and difficulties arising in the construction of fractional-dynamic model analogs of standard models by using fractional calculus. This stems from the fact that some fundamental properties of fractional derivatives (such as the multiplicity principle, the solvability and correspondence principles, and the interpretability principle) violate various known standard rules and properties that are fulfilled for derivatives of integer order.
Information retention (or so-called memory effect) is one of the most basic properties of fractional-order derivatives. This significant property has made these operators one of the most efficient tools in computational models arising in biology. In other words, the basic information of the model from the beginning of time is utilized to characterize the behavior of the phenomenon at any desired time. The use of fractional derivatives has made it possible to employ such a valuable feature. To take advantage of memory-related benefits in the context of fractional-order derivatives, our main objective is to study a nonlinear system of the delayed-fractional model in studying HFMD. For this purpose, the present contribution is structured as follows: Some mathematical background, mainly about recent definitions on fractional calculus model, is formulated in Sect. 2. The proposed model is introduced in Sect. 3. In Sect. 4, we investigate some mathematical frameworks of the model, including the equilibrium points, the basic reproduction number, and the existence and uniqueness of the model’s solution. Then, we present some corresponding numerical simulations in Sect. 5. Finally, some conclusions are drawn in the last section of the article.
Some basic preliminaries on fractional operators
Employing new definitions in mathematics always makes extensive progress in modeling various phenomena around us in the world. One of these new and powerful topics is the use of fractional calculus concepts. In this section, we will have an overview of some of known basic definitions corresponding to fractional calculus that are used in what follows. For this purpose, we present the definition of Riemann–Liouville fractional integral and derivative operators and of order , respectively. Then, we define the Caputo fractional derivative of order as a modification of the Riemann–Liouville fractional derivative. Further, we present some properties of the Mittag-Leffler function , (see [48]).
Definition 1
For a given integrable function , the fractional integral operator in the Riemann–Liouville sense of order is given by [36]
| 1 |
where denotes the well-known gamma function.
Definition 2
For a given function in , the fractional derivative operator in the Riemann–Liouville sense of order is given by
Definition 3
Let and . The Caputo fractional derivative operator of order is defined by
The following two important propositions are consequences of the above definitions:
Definitions (2) and (3) are different from each other, and the relation between the two types of fractional derivatives is as follows:
The Caputo derivative has the main advantage that the initial condition of the corresponding problem has the same value as the ordinary differential equation. Moreover, for a constant-valued function, the Caputo derivative is zero.
Definition 4
The Mittag-Leffler function of two parameters is given by
| 2 |
where , denotes the complex plane.
For , the Mittag-Leffler function gives the exponential function . Moreover, the Mittag-Leffler function satisfies the following useful equality:
Lemma 1
For and , we obtain
| 3 |
Also,
| 4 |
where .
Definition 5
The AB-Caputo derivative operator of is defined as follows [9]:
| 5 |
and .
Definition 6
The AB-Caputo integral operator of order α is defined as
| 6 |
Lemma 2
For the operators defined in Equations (6) and (5), the following union is established [2]:
| 7 |
The delayed-fractional version of the model
In recent years, modeling real-world problems using the fractional delay differential equations (FDDEs) has attracted much attention among mathematicians, physicists, and engineers. Due to the wide application of these equations, the theoretical and practical aspects of this category of equations have been extensively studied by researchers in many research articles such as [42, 44, 49].
In the light of these facts and after employing the AB-Caputo fractional derivative and defining two-time delays and in the model presented in [42], we arrive at the following FDDE for the spread of the disease:
| 8 |
where , and subject to given initial conditions
The effective populations in this model are categorized into six state variables as follows: the susceptible subpopulation , the treated and vaccinated subpopulation , the clinically infectious subpopulation , the recovered subpopulation , the vaccinated subpopulation , and finally, the vaccinated carrier subpopulation . We symbolize the sum of all subpopulations by . In this model, the transmission rate is denoted by β. Moreover, b is the birth rate, represents the transforming rate from to . Also, is utilized to describe the vaccinated carrier rate, ω is the recovery rate, ϕ is the rate of protection loss, and are two delay parameters. Moreover, μ denotes the natural death rate, treating and vaccinating rate of susceptible people is shown by , culling of clinical infective, and vaccinated carrier rate is denoted by δ. Finally, the rates corresponding to the treating vaccinated, vaccinating susceptible, and recovery of treated people are ϵ, , and , respectively [29].
In consecutive subsections, we analyze some theoretical aspects of the fractional model outlined in (8).
The equilibrium points
The equilibrium points of the system are determined as follows:
- The disease-free equilibrium of the model is , where
and9 - The endemic equilibrium of the model is , where
and10 11
A detailed survey of the stability of these equilibrium points can be found in the reference [42].
The basic reproduction number
Employing the next generation matrix method [14], the basic reproduction number of model (8) is given by [42]
| 12 |
Existence of the solution
To investigate and prove the existence of a solution for the model, we can first apply the AB-Caputo integral operator (6) to the sides of model (8) to give the following equalities:
| 13 |
where , and
| 14 |
Defining , and , Equation (13) can be considered as follows:
| 15 |
The following iterative process can be defined:
| 16 |
Subtracting two consecutive terms gives
| 17 |
For simplicity, we set . Hence, one has
| 18 |
Thus, we get
| 19 |
Hence
Whenever N satisfies the Lipschitz condition with respect to F, thus
Hence the following inequality will be obtained:
Now, replacing by its value, we get
Also, we have
And finally,
| 20 |
By choosing
| 21 |
we are now in a position to define the following sequence for :
| 22 |
where when . Thus,
| 23 |
Now, we can write
Taking the standard norm on both sides of the above equation, we conclude that
If , the right-hand side of the equation tends to zero. So, one gets
| 24 |
And that was what we were trying to prove in this subsection.
The uniqueness of the solution
In this part, we are looking for the proof of the uniqueness of the solution related to the model. To do this, let us consider that model admits two solutions and . Then we can write
| 25 |
| 26 |
| 27 |
If holds, we get , from which the equality results. This means that the system has a unique solution.
A numerical method
In recent years, various approximate methods have been used to solve the system of fractional-order differential equations. In each of these methods, a specific idea is used to discretize the problem and approximate the solution of the system.
In order to express the method, we first need to consider a fractional delay differential equation given by
| 28 |
To determine the numerical method, we first consider the domain discretization as follows:
Keeping these notations in mind, we obviously have , .
In other words, it can be written
The main assumption of the numerical method is that we have approximated the value as the solution function in points
and now we are looking for the value of .
The next idea used is to apply the definition of the integral operator (6), which results in the following relation:
| 29 |
Setting in (29) gives
| 30 |
Now, we utilize the product trapezoidal quadrature formula to approximate (30). Then, the corrector approximation for is obtained as follows:
| 31 |
where , , and
| 32 |
The unknown term that appears in (31) is also calculated using the product rectangle rule as follows:
| 33 |
where
| 34 |
Simulation results and discussion
In this section, the numerical simulations corresponding to the proposed model along with the attached approximate method are examined. In all graphs, the following values are used as the default of the constants, unless we intentionally change some of these values in the graphs ourselves. These changes are expressed in each graph.
| 35 |
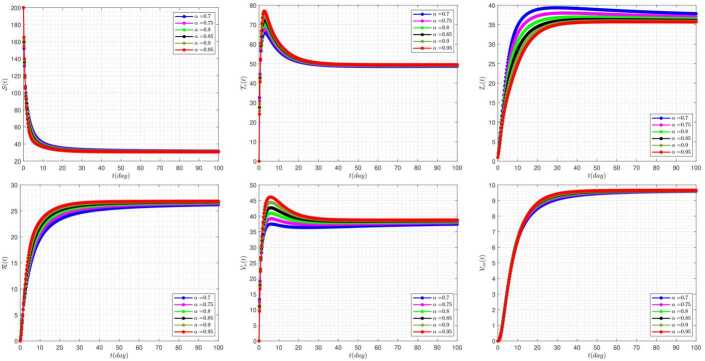
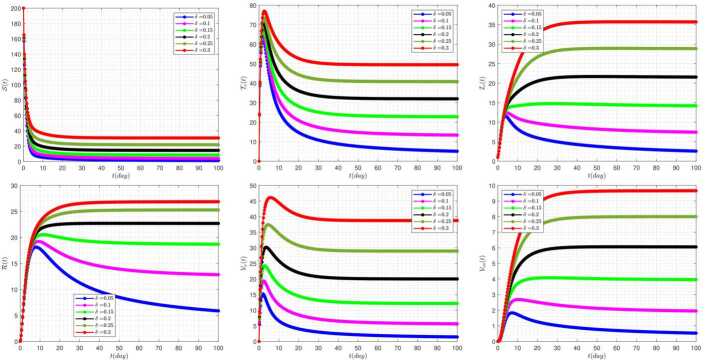
The effect of fractional-order parameter (α) on the disease evolution, in the absence of delay parameters , is shown in Fig. 1. It is seen that when α increases, then the susceptible and clinically infected classes decrease, and treated and vaccinated, recovered, vaccinated, and vaccinated carrier classes increase. In this case, all model solutions converge to the internal equilibrium point of the system.
Figure 1.
The impact of α on the results for
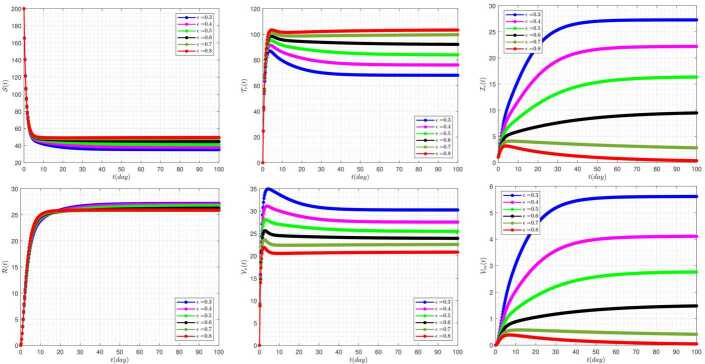
The effect of the treating of vaccinated people rate (ϵ) on the disease prevalence, in the absence of delay parameters , is presented in Fig. 2. In some diagrams of this figure, it can be seen that a change in the value of the ϵ parameter can cause very significant changes in the overall condition of the system. In a way, by taking some values, the behavior of some solutions will be completely different from others in general. For example, by taking , the graphs related to the two solutions clinically infected classes and vaccinated carrier classes take on a damping state and tend to zero.
Figure 2.
The impact of ϵ on the results for and
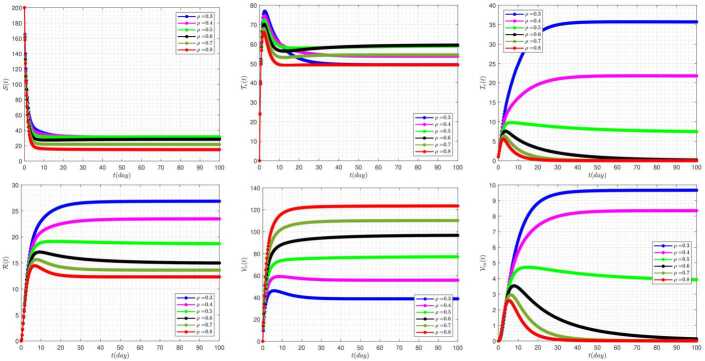
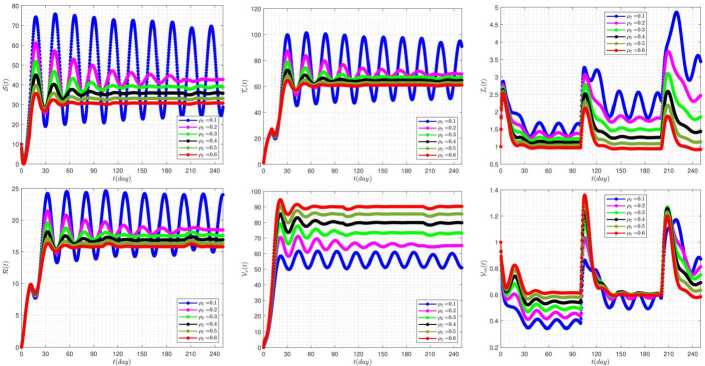
Further, the effect of the rate of vaccination (ρ) on the disease evolution, in the absence of delay parameters , is displayed in Fig. 3. In this figure it is clear that by taking , and 0.8 the graphs related to the two solutions clinically infected classes and vaccinated carrier classes take on a damping state and tend to zero. In other words, in these cases, the system moves to the disease-free equilibrium point. These conditions are fully consistent with the theoretical arguments presented in this paper and reference [42].
Figure 3.
The impact of ρ on the results for and
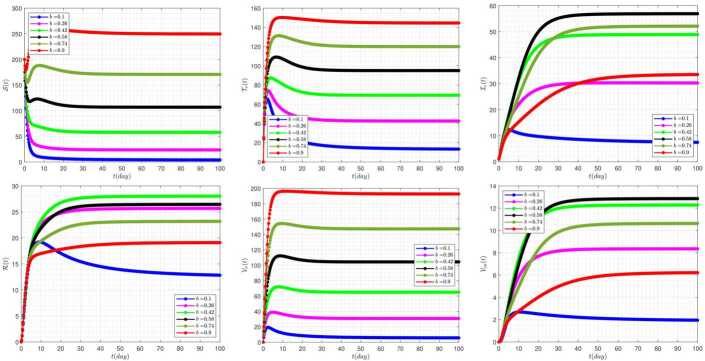
The effect of the rate of vaccination (b) on the disease dynamics, in the absence of delay parameters , has been plotted in Fig. 4. The diagrams in this figure clearly show that the curves corresponding to always create the lowest values in all possible solutions in the system. Taking the value stated in this case into account the system moves towards stability at the endemic equilibrium point .
Figure 4.
The impact of b on the results for and
To investigate the significant effect of the rate of culling of clinical infective and vaccinated carrier people (δ) on the disease dynamics, in the absence of delay parameters , we have presented Fig. 5. A distinctive feature of the diagrams in this figure is that the final values for system solutions always have an alignment correlation to the parameter δ. In other words, by increasing the value of parameter δ, the equilibrium points of the system are placed in higher levels.
Figure 5.
The impact of δ on the results for and
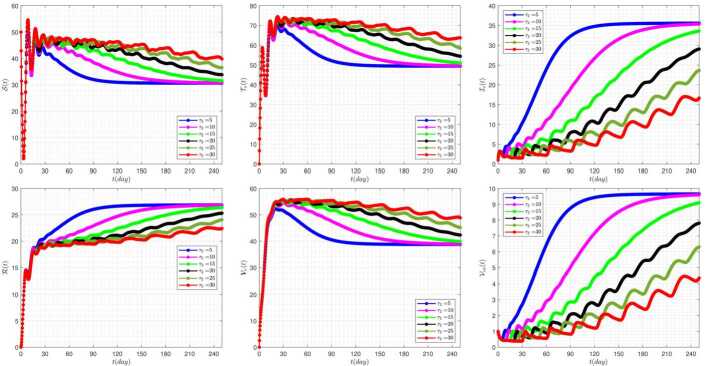
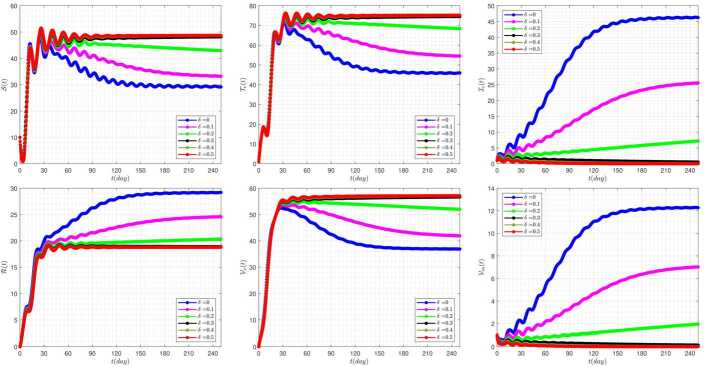
Figure 6 exhibits the effect of the rate of delay parameter () on the disease dynamics of the model when we take . As we have seen in this figure, by increasing the delay parameter , the solutions , , have similar trends. The same is true also for , , . Also, by increasing the value of the parameter , the system shows more oscillating behavior.
Figure 6.
The impact of on the results for and
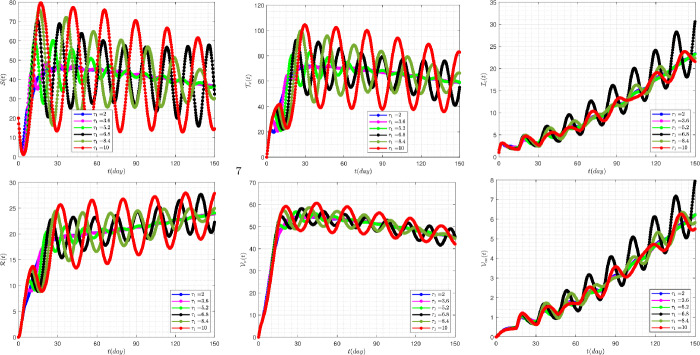
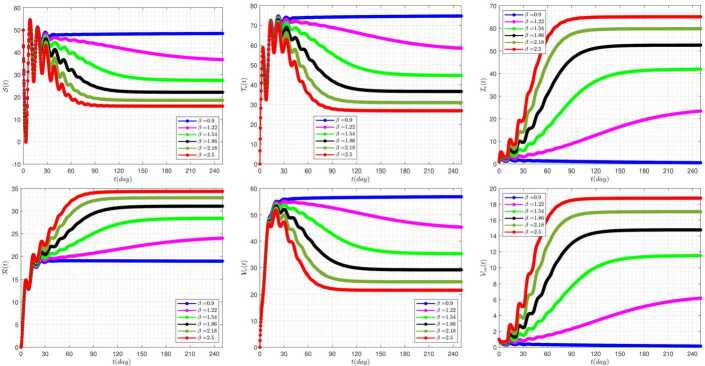
Figure 7 demonstrates the effect of the rate of delay parameter () on the disease variation of the model when we take . The occurrence of oscillating properties in this figure is very obvious. In addition, by increasing the value of the delay parameter , the system reveals more severe instability behavior.
Figure 7.
The impact of on the results for and
Figure 8 demonstrates the effect of the rate of vaccinating susceptible people () on the disease dynamics for delay parameters , . In this figure, the high sensitivity of the model’s response behavior to the parameter can be seen. For example, by considering , the behavior of the system is quite volatile and unstable. While for the bigger values of this parameter, the system’s response show more stable behavior.
Figure 8.
The impact of on the results for , , and
The effect of the rate of culling of clinical infective and vaccinated carrier people (δ) on the disease dynamics, for delay parameters , , has been displayed in Fig. 9.
Figure 9.
The impact of δ on the results for , , and
The influence of the transmission rate (β) on the disease dynamics, for delay parameters , , is investigated in Fig. 10. It can be seen that for the , the system tends toward the disease-free equilibrium , and for the other considered values to the endemic equilibrium point of . Therefore, β can play a very important and constructive role in the behavior and dynamics of the disease.
Figure 10.
The impact of β on the results for , , and
Conclusion
Humans have been exposed to infectious diseases throughout history and have always tried to be able to control the spread of the disease and then find an effective treatment process to treat the disease. One way to control infectious diseases is to study the dynamic systems that describe the extent and prevalence of the disease. On the other hand, applying the modern concepts presented in the study of these dynamic systems can lead to dramatic advances in the study and control of this disease. In this article, we examined the prevalence of hand-foot-mouth disease in a certain population that has been described by a fractional system of ordinary differential equations. The model is constructed using the Atangana–Baleanu fractional derivative and two constant parameters to apply the time delay in the solutions. In order to take advantage of the concept of memory in the evolution of the model, we have employed a well-known derivative with fractional order as well as two delay parameters in the model.
Several numerical simulations revealed the effects of the fractional parameter, and the existence of two delay parameters in the disease dynamic. Through some performed experiments, we also investigated the sensitivity analysis of the model to some numerical parameters of the model. In some cases, it was observed that with a slight change in some of these parameters, very fundamental changes in the behavior of system responses occur. Another noteworthy aspect of this paper is the high degree of influence of the model on the delay parameters. For some specific values for the parameters in the system, unstable chaotic behavior can be observed in the system responses. The results of the model presented in this paper provide the ability to investigate the effects of time delay parameters, prophylactic vaccination, reactive vaccination, prophylactic treatment, and reaction-reflecting parameters on disease outbreak in a population. The process presented in this article can be applied to other infectious disease models.
Acknowledgments
Acknowledgements
We would like to express our special thanks to the editor and anonymous referees for their valuable suggestions and advisory comments on improving the quality of this paper.
Availability of data and materials
Not applicable.
Authors’ contributions
All authors contributed equally and significantly in writing this paper. All authors have read and approved the final paper.
Funding
This research work is not supported by any funding agencies.
Competing interests
The authors declare that they have no competing interests.
References
- 1.https://www.cdc.gov/hand-foot-mouth/about/signs-symptoms.html
- 2. Abdeljawad, T., Baleanu, D.: Integration by parts and its applications of a new nonlocal fractional derivative with Mittag-Leffler nonsingular kernel (2016). arXiv:1607.00262. Preprint
- 3.Akgül A. A novel method for a fractional derivative with non-local and non-singular kernel. Chaos Solitons Fractals. 2018;114:478–482. doi: 10.1016/j.chaos.2018.07.032. [DOI] [Google Scholar]
- 4.Akgül E.K. Solutions of the linear and nonlinear differential equations within the generalized fractional derivatives. Chaos, Interdiscip. J. Nonlinear Sci. 2019;29(2):023108. doi: 10.1063/1.5084035. [DOI] [PubMed] [Google Scholar]
- 5.Atangana A. Blind in a commutative world: simple illustrations with functions and chaotic attractors. Chaos Solitons Fractals. 2018;114:347–363. doi: 10.1016/j.chaos.2018.07.022. [DOI] [Google Scholar]
- 6.Atangana A. Fractional discretization: the African’s tortoise walk. Chaos Solitons Fractals. 2020;130:109399. doi: 10.1016/j.chaos.2019.109399. [DOI] [Google Scholar]
- 7.Atangana A., Akgül A. Can transfer function and Bode diagram be obtained from Sumudu transform. Alex. Eng. J. 2020;59(4):1971–1984. doi: 10.1016/j.aej.2019.12.028. [DOI] [Google Scholar]
- 8.Atangana A., Akgül A., Owolabi K.M. Analysis of fractal fractional differential equations. Alex. Eng. J. 2020;59(3):1117–1134. doi: 10.1016/j.aej.2020.01.005. [DOI] [Google Scholar]
- 9. Atangana, A., Baleanu, D.: New fractional derivatives with nonlocal and non-singular kernel: theory and application to heat transfer model (2016). arXiv:1602.03408. Preprint
- 10.Atangana A., Gómez-Aguilar J. Decolonisation of fractional calculus rules: breaking commutativity and associativity to capture more natural phenomena. Eur. Phys. J. Plus. 2018;133(4):166. doi: 10.1140/epjp/i2018-12021-3. [DOI] [Google Scholar]
- 11.Baleanu D., Fernandez A., Akgül A. On a fractional operator combining proportional and classical differintegrals. Mathematics. 2020;8(3):360. doi: 10.3390/math8030360. [DOI] [Google Scholar]
- 12.Chan S.J., Labadin J., Podin Y. A dynamic SEIPR model for the spread of hand, foot and mouth disease in Sarawak. J. Telecommun. Electron. Comput. Eng. 2017;9(3–10):125–129. [Google Scholar]
- 13.Chen S., Yang D., Liu R., Zhao J., Yang K., Chen T. Estimating the transmissibility of hand, foot, and mouth disease by a dynamic model. Publ. Health. 2019;174:42–48. doi: 10.1016/j.puhe.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Diekmann O., Heesterbeek J.A.P., Metz J.A. On the definition and the computation of the basic reproduction ratio R_0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990;28(4):365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 15.Djilali S., Ghanbari B. Coronavirus pandemic: a predictive analysis of the peak outbreak epidemic in South Africa, Turkey, and Brazil. Chaos Solitons Fractals. 2020;138:109971. doi: 10.1016/j.chaos.2020.109971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Z., Zhang W., Zhang D., Yu S., Hao Y. Estimating the basic reproduction rate of HFMD using the time series SIR model in Guangdong, China. PLoS ONE. 2017;12(7):e0179623. doi: 10.1371/journal.pone.0179623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu X., Wan Z., Li Y., Hu Y., Jin X., Zhang C. National epidemiology and evolutionary history of four hand, foot and mouth disease-related enteroviruses in China from 2008 to 2016. Virol. Sin. 2020;35(1):21–33. doi: 10.1007/s12250-019-00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghanbari B. On forecasting the spread of the COVID-19 in Iran: the second wave. Chaos Solitons Fractals. 2020;140:110176. doi: 10.1016/j.chaos.2020.110176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghanbari B., Atangana A. A new application of fractional Atangana–Baleanu derivatives: designing abc-fractional masks in image processing. Phys. A, Stat. Mech. Appl. 2020;542:123516. doi: 10.1016/j.physa.2019.123516. [DOI] [Google Scholar]
- 20.Ghanbari B., Atangana A. Some New Edge Detecting Techniques Based on Fractional Derivatives with Non-local and Non-singular Kernels. Berlin: Springer; 2020. pp. 1–19. [Google Scholar]
- 21.Ghanbari B., Djilali S. Mathematical and numerical analysis of a three-species predator-prey model with herd behavior and time fractional-order derivative. Math. Methods Appl. Sci. 2020;43(4):1736–1752. doi: 10.1002/mma.5999. [DOI] [Google Scholar]
- 22.Ghanbari B., Gómez-Aguilar J. Modeling the dynamics of nutrient–phytoplankton–zooplankton system with variable-order fractional derivatives. Chaos Solitons Fractals. 2018;116:114–120. doi: 10.1016/j.chaos.2018.09.026. [DOI] [Google Scholar]
- 23.Ghanbari B., Gómez-Aguilar J. Analysis of two avian influenza epidemic models involving fractal-fractional derivatives with power and Mittag-Leffler memories. Chaos, Interdiscip. J. Nonlinear Sci. 2019;29(12):123113. doi: 10.1063/1.5117285. [DOI] [PubMed] [Google Scholar]
- 24.Ghanbari B., Kumar D. Numerical solution of predator-prey model with Beddington–DeAangelis functional response and fractional derivatives with Mittag-Leffler kernel. Chaos, Interdiscip. J. Nonlinear Sci. 2019;29(6):063103. doi: 10.1063/1.5094546. [DOI] [PubMed] [Google Scholar]
- 25.Ghanbari B., Kumar S., Kumar R. A study of behaviour for immune and tumor cells in immunogenetic tumour model with non-singular fractional derivative. Chaos Solitons Fractals. 2020;133:109619. doi: 10.1016/j.chaos.2020.109619. [DOI] [Google Scholar]
- 26.Gu J., Liang L., Song H., Kong Y., Ma R., Hou Y., Zhao J., Liu J., He N., Zhang Y. A method for hand-foot-mouth disease prediction using geodetector and LSTM model in Guangxi, China. Sci. Rep. 2019;9(1):1–10. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halder N., Roy N. Compartmental modeling of hand, foot and mouth infectious disease (HFMD) Res. J. Appl. Sci. 2010;5(3):1–6. [Google Scholar]
- 28.Ji T., Han T., Tan X., Zhu S., Yan D., Yang Q., Song Y., Cui A., Zhang Y., Mao N., et al. Surveillance, epidemiology, and pathogen spectrum of hand, foot, and mouth disease in mainland of China from 2008 to 2017. Biosafety Health. 2019;1(1):32–40. doi: 10.1016/j.bsheal.2019.02.005. [DOI] [Google Scholar]
- 29.Keeling M.J., Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton: Princeton University Press; 2011. [Google Scholar]
- 30.Kheiri H., Jafari M. Fractional optimal control of an HIV/AIDS epidemic model with random testing and contact tracing. J. Appl. Math. Comput. 2019;60(1–2):387–411. doi: 10.1007/s12190-018-01219-w. [DOI] [Google Scholar]
- 31.Lai C.C., Jiang D.S., Wu H.M., Chen H.H. A dynamic model for the outbreaks of hand, foot, and mouth disease in Taiwan. Epidemiol. Infect. 2016;144(7):1500–1511. doi: 10.1017/S0950268815002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen N.T., Pham H.V., Hoang C.Q., Nguyen T.M., Nguyen L.T., Phan H.C., Phan L.T., Vu L.N., Minh N.N.T. Epidemiological and clinical characteristics of children who died from hand, foot and mouth disease in Vietnam, 2011. BMC Infect. Dis. 2014;14(1):341. doi: 10.1186/1471-2334-14-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni X., Li X., Xu C., Xiong Q., Xie B., Wang L., Peng Y., Li X. Risk factors for death from hand–foot–mouth disease: a meta-analysis. Epidemiol. Infect. 2020;148:e44. doi: 10.1017/S0950268819002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nistal R., De la Sen M., Alonso-Quesada S., Ibeas A. Supervising the vaccinations and treatment control gains in a discrete SEIADR epidemic model. Int. J. Innov. Comput. Inf. Control. 2019;15:2053–2067. [Google Scholar]
- 35.Owolabi K.M., Atangana A., Akgul A. Modelling and analysis of fractal-fractional partial differential equations: application to reaction-diffusion model. Alex. Eng. J. 2020;59(4):2477–2490. doi: 10.1016/j.aej.2020.03.022. [DOI] [Google Scholar]
- 36.Podlubny I. Fractional Differential Equations: An Introduction to Fractional Derivatives, Fractional Differential Equations, to Methods of Their Solution and Some of Their Applications. Amsterdam: Elsevier; 1998. [Google Scholar]
- 37.Rahman G., Nisar K.S., Ghanbari B., Abdeljawad T. On generalized fractional integral inequalities for the monotone weighted Chebyshev functionals. Adv. Differ. Equ. 2020;2020(1):1. doi: 10.1186/s13662-020-02830-7. [DOI] [Google Scholar]
- 38.Sarma N., Chakraborty S., Dutta A., Sadhukhan P.C. Hand, foot and mouth disease in West Bengal, India: a preliminary report on clinicovirological trend over 3 successive years (2013–2015) Indian J. Dermatol. 2017;62(5):486–490. doi: 10.4103/ijd.IJD_381_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi R., Lu T. Dynamic analysis and optimal control of a fractional order model for hand-foot-mouth disease. J. Appl. Math. Comput. 2020;64(1–2):565–590. doi: 10.1007/s12190-020-01369-w. [DOI] [Google Scholar]
- 40.Tan H., Cao H. The dynamics and optimal control of a hand-foot-mouth disease model. Comput. Math. Methods Med. 2018;2018:9254794. doi: 10.1155/2018/9254794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarasov V.E. Rules for fractional-dynamic generalizations: difficulties of constructing fractional dynamic models. Mathematics. 2019;7(6):554. doi: 10.3390/math7060554. [DOI] [Google Scholar]
- 42.Tessema K.M., Chirove F., Sibanda P. Modeling control of foot and mouth disease with two time delays. Int. J. Biomath. 2019;12(04):1930001. doi: 10.1142/S179352451930001X. [DOI] [Google Scholar]
- 43.Toufik M., Atangana A. New numerical approximation of fractional derivative with non-local and non-singular kernel: application to chaotic models. Eur. Phys. J. Plus. 2017;132(10):444. doi: 10.1140/epjp/i2017-11717-0. [DOI] [Google Scholar]
- 44.Wang H., Yu Y., Wen G., Zhang S., Yu J. Global stability analysis of fractional-order Hopfield neural networks with time delay. Neurocomputing. 2015;154:15–23. doi: 10.1016/j.neucom.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 45.Wang J., Hu T., Sun D., Ding S., Carr M.J., Xing W., Li S., Wang X., Shi W. Epidemiological characteristics of hand, foot, and mouth disease in Shandong, China, 2009–2016. Sci. Rep. 2017;7(1):1–9. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organization, et al.: A guide to clinical management and public health response for hand. foot and mouth disease (HFMD) (2011)
- 47.Yang Z., Hao J., Huang S., Yang W., Zhu Z., Tian L., Lu Y., Xiang H., Liu S. Acute effects of air pollution on the incidence of hand, foot, and mouth disease in Wuhan, China. Atmos. Environ. 2020;225:117358. doi: 10.1016/j.atmosenv.2020.117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasar B.Y. Generalized Mittag-Leffler function and its properties. New Trends Math. Sci. 2015;3(1):12–18. [Google Scholar]
- 49.Yuan L., Yang Q., Zeng C. Chaos detection and parameter identification in fractional-order chaotic systems with delay. Nonlinear Dyn. 2013;73(1–2):439–448. doi: 10.1007/s11071-013-0799-6. [DOI] [Google Scholar]
- 50.Zhang X., Xu C., Xiao G. Space-time heterogeneity of hand, foot and mouth disease in children and its potential driving factors in Henan, China. BMC Infect. Dis. 2018;18(1):638. doi: 10.1186/s12879-018-3546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J., Jiang F., Zhong L., Sun J., Ding J. Age patterns and transmission characteristics of hand, foot and mouth disease in China. BMC Infect. Dis. 2016;16(1):691. doi: 10.1186/s12879-016-2008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.