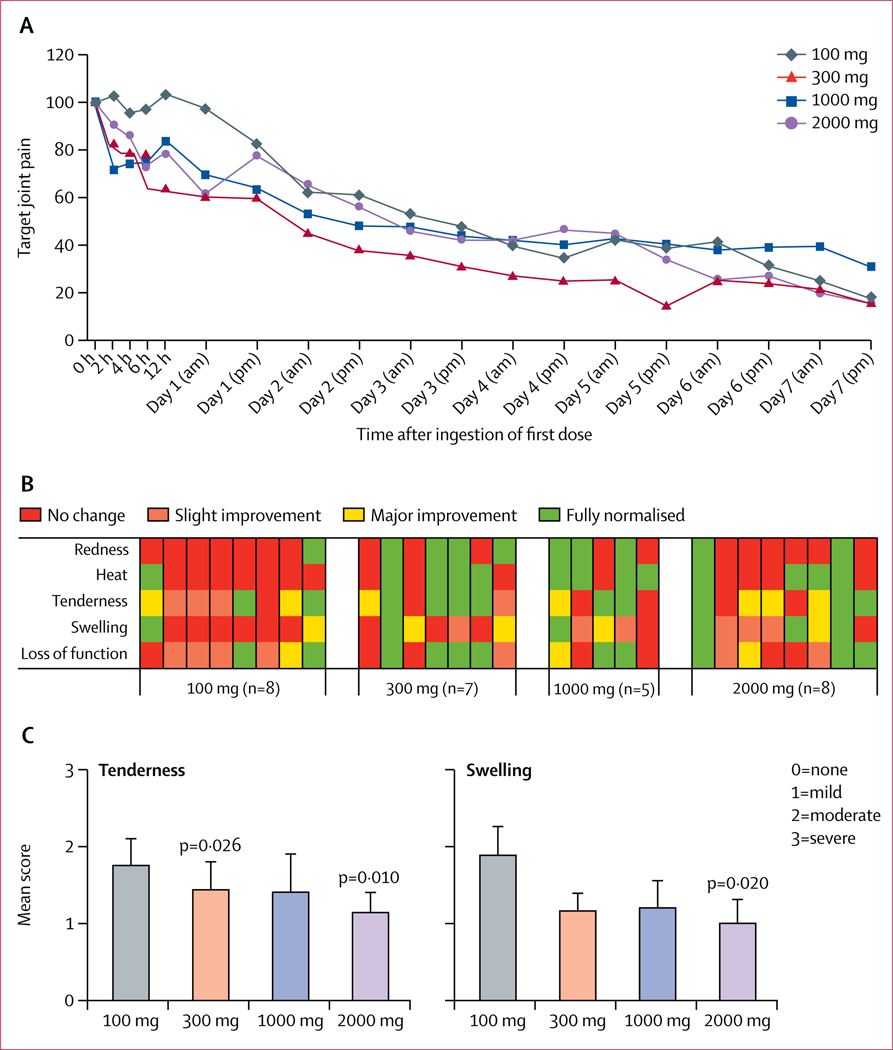
Figure 2: Target joint pain and joint inflammation.
A) Target joint pain for all timepoints. Patients scored target joint pain on a 0–100 mm VAS scale twice daily. Data are presented as mean target joint pain in comparison with the pain score at baseline, which was set at 100%.
(B) Investigator-assessed signs of inflammation. At day 3 a score graded from no change to slight improvement, major improvement, or fully normalised was given for the target joint for all five components of inflammation. Each column represents one individual (one patient in the 1000 mg/day group missed the day 3 visit).
(C) Investigator-scored tenderness at day 3 (mean ± SEM; 0=no pain, 1=mild pain, 2=moderate pain, 3=severe pain), and investigator-scored swelling in target joint at day 3 (mean ± SEM; 0=no swelling, 1=mild swelling, 2=moderate swelling, 3=severe swelling). VAS=visual analogue scale.